FDA Approvals - Cancer Currents Blog
News on recent approvals of cancer therapies by the Food and Drug Administration. Includes expert comments on how the approval will influence patient care and future research.
-
FDA Approval Likely to Change Initial Treatment for Some People with Advanced Colorectal Cancer
FDA has approved the combination of the immunotherapy drugs ipilimumab (Yervoy) and nivolumab (Opdivo) for the initial treatment of people with advanced colorectal cancer whose tumors are classified as MSI-H or dMMR.
-
Zenocutuzumab Approved to Treat Lung and Pancreatic Cancers with Rare Genetic Change
FDA has approved zenocutuzumab (Bizengri) to treat people with pancreatic or non-small cell lung cancer whose tumors have a rare genetic alteration called an NRG1 fusion. The approval is based on a clinical trial in which the drug shrank tumors in a third of patients.
-
FDA Approves Injectable Nivolumab, an Alternative to IV Infusion
The injectable form of nivolumab, called Opdivo Qvantig, is quicker and easier to give, several oncologists said, and is just as effective as the intravenous form. Injectable forms of other immunotherapies are also on the horizon.
-
FDA Approvals Expand Initial Treatment Options for Multiple Myeloma
FDA’s approvals of Darzalex Faspro and Sarclisa, each used in combination with standard three-drug treatment regimens, should change the initial treatment of newly diagnosed multiple myeloma, including for patients who can’t get a stem cell transplant.
-
Colorectal Cancer Screening: Where Does the Shield Liquid Biopsy Fit In?
FDA recently approved the Shield test, the first blood test for the primary screening of people at average risk of colorectal cancer. Where does it fit in with other screening options for the disease, including colonoscopy and stool tests?
-
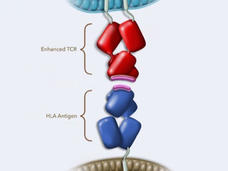
FDA Approves Engineered Cell Therapy for Advanced Synovial Sarcoma
FDA approved afami-cel (Tecelra) to treat metastatic synovial sarcoma, a type of soft tissue sarcoma. The approval is for patients who have already received chemo and whose tumors are positive for MAGE-A4. Afami-cel is the first T-cell receptor therapy approved for cancer.
-
More Immunotherapy Options Approved for Treating Endometrial Cancer
People with advanced endometrial cancer now have new FDA-approved treatment options: pembrolizumab and durvalumab, paired with chemotherapy, for tumors with a genetic change called mismatch repair deficiency. The agency also expanded the approved uses of dostarlimab for the disease.
-
Tovorafenib Approved for Some Children with Low-Grade Glioma
FDA has granted an accelerated approval to tovorafenib (Ojemda) for kids and teens who have low-grade glioma with changes in the BRAF gene. In a small clinical trial, the drug shrank or completely eliminated tumors in nearly 70% of patients.
-
Alectinib Approved as an Adjuvant Treatment for Lung Cancer
FDA has approved alectinib (Alecensa) as adjuvant therapy for people with lung cancer who have ALK-positive tumors. In a clinical trial, alectinib helped people live longer after surgery without their cancer returning than chemotherapy.
-
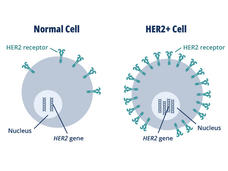
FDA Approves Trastuzumab Deruxtecan for Any HER2-Positive Solid Cancer
Trastuzumab deruxtecan (Enhertu) can now be used to treat any advanced solid cancer that produces high levels of the protein HER2 (HER2-positive tumors). FDA’s accelerated approval was based on findings from three clinical trials.
-
Approval of Elahere Expands Treatment Options for Some Advanced Ovarian Cancers
FDA approved mirvetuximab soravtansine-gynx (Elahere) to treat people with advanced, platinum-resistant ovarian cancer whose tumors overproduce a protein called FR-α. The full approval was based on the results of a large, randomized trial called MIRASOL, which showed Elahere improved survival for these people.
-
First Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma
In an event more than three decades in the making, FDA has approved lifileucel (Amtagvi), the first cancer treatment that uses immune cells called tumor-infiltrating lymphocytes, or TILs.
-
Repotrectinib Expands Treatment Options for Lung Cancers with ROS1 Fusions
The results of the clinical trial that led to FDA’s 2023 approval of repotrectinib (Augtyro) for lung cancers with ROS1 fusions have been published. The drug shrank tumors in 80% of people receiving the drug as an initial treatment.
-
Enzalutamide Gets Added Approval for Prostate Cancer That Hasn’t Spread
Under a new FDA approval, enzalutamide (Xtandi) can now be used alone, or in combination with leuprolide, to treat people with nonmetastatic prostate cancer that is at high risk of returning after surgery or radiation.
-
Toripalimab Becomes First Immunotherapy Drug Approved for Nasopharyngeal Cancer
FDA approved toripalimab (Loqtorzi) based on the results of a large clinical trial showing that, when added to chemotherapy, the drug extended survival in people with nasopharyngeal cancer that returned after initial treatment or spread in the body.
-
FDA Amends Approval of Pembrolizumab to Treat Stomach and Esophageal Junction Cancer
FDA has changed its 2021 approval of pembrolizumab (Keytruda) along with trastuzumab (Herceptin) and chemotherapy for treating HER2-positive stomach or GEJ cancer. The agency also announced a new approval of pembrolizumab for HER2-negative forms of these same cancers.
-
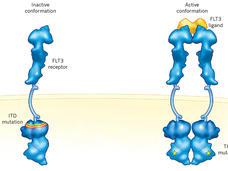
Quizartinib Approval Adds New Treatment Option for AML, Including in Older Patients
Treatment options for people with acute myeloid leukemia (AML) have expanded yet again. On July 20, FDA approved quizartinib (Vanflyta) combined with chemotherapy as a first-line treatment for AML with a specific change in the FLT3 gene.
-
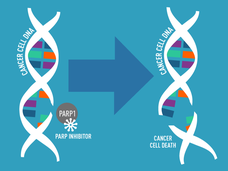
FDA Approves New Initial Treatment Option for Some Metastatic Prostate Cancers
FDA approved enzalutamide (Xtandi) combined with talazoparib (Talzenna) for metastatic castration-resistant prostate cancer with alterations in any of 12 DNA repair genes. The drug combination, which blocks both DNA repair activities and hormones that fuel cancer growth, was more effective than the standard treatment in a large clinical trial.
-
Tucatinib and Trastuzumab Combination Approved for Advanced Colorectal Cancer
FDA approved tucatinib (Tukysa) with trastuzumab (Herceptin) to treat HER2-positive advanced colorectal cancer. The approval was based on the MOUNTAINEER trial, in which nearly 40% of participants’ tumors shrank after receiving the drug combination.
-
Zanubrutinib’s Approval Improves Targeted Treatment for CLL
FDA has approved zanubrutinib (Brukinsa) for chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) based on results from two clinical trials. In both trials, the drug, which blocks a protein called BTK, was more effective and caused fewer side effects than other treatments.