Tucatinib and Trastuzumab Combination Approved for Advanced Colorectal Cancer
, by Sharon Reynolds
Some people with colorectal cancer that can’t be removed surgically or has spread elsewhere in the body have a new treatment option. On January 19, the Food and Drug Administration (FDA) granted accelerated approval to the combination of two targeted drugs, tucatinib (Tukysa) and trastuzumab (Herceptin) for people with advanced colorectal cancer that produces an excess amount of a protein called HER2.
To be eligible to receive the new combination, people’s tumors must also not have changes in a group of genes called RAS, and people must have previously received at least two standard treatments, including chemotherapy.
In the clinical trial that led to the accelerated approval, called MOUNTAINEER, 38% of people who received the drug combination had their tumors shrink or disappear. In another 33%, tumors stopped growing for some time. At the time the study results were presented last July, more than half the participants who received the drugs were still alive 2 years after beginning treatment.
The available treatment options for people whose metastatic colorectal cancer has returned or started growing again after receiving standard treatments are not very effective, explained John Strickler, M.D., of Duke University, who led the trial.
“That [approach] has a response rate of less than 5%, and generally controls disease for around 2 to 4 months,” Dr. Strickler said. “So this treatment represents a fairly substantial breakthrough for patients who have HER2-positive disease” that has returned or started growing again.
HER2-positive tumors make up a small minority of colorectal cancers: only about 3% overall. Focusing on smaller and smaller groups of patients with targeted therapies can make recruiting enough people to run clinical trials challenging, said Carmen Allegra, M.D., who works with NCI’s Cancer Therapy Evaluation Program and was not involved with the study.
“But when you do find something that helps small subsets of patients, the results are often quite impressive like this,” Dr. Allegra said
Cutting off fuel for cancer cells
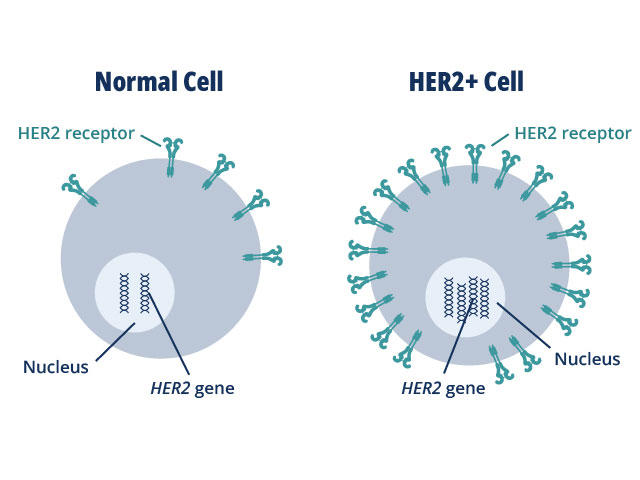
HER2 plays a role in normal cell growth. In many cancer types, tumor cells make extra copies of the gene that produces the HER2 protein, known as gene amplification.
The resulting flood of HER2 protein causes cancer cells to grow uncontrollably. But, on the flip side, cancer cells may also become dependent on this extra HER2 and cutting off the flow of the protein can cause them to stop growing or die.
HER2's role is best understood in breast cancer, where up to 30% of tumors overexpress HER2 and HER2-targeted treatments are commonly used. In the last few years, researchers have discovered that HER2 is also overproduced in some stomach and esophageal cancers, Dr. Strickler said, and researchers are teasing out its contribution to other cancer types.
More and more, people with newly diagnosed metastatic colorectal cancer undergo certain types of gene sequencing, explained Dr. Strickler. This sequencing looks for gene changes that can be targeted with approved drugs, as well as mutations that can predict resistance to certain treatments.
“Increasingly, when we were sequencing tumors to look for mutations, we were incidentally finding HER2 amplification. But we had no FDA-approved therapies targeting HER2” for colorectal cancer, Dr. Strickler said.
He and his colleagues began a small study of two drugs targeting HER2: trastuzumab, which has been the backbone of treatment for HER2-postive breast cancer, and tucatinib, a newer drug which has proven to be particularly effective in combination with trastuzumab in breast cancer.
In studies in mice, “it had been shown repeatedly that these drugs work better when given together,” Dr. Strickler said. “Each drug by itself has modest antitumor effects in colorectal cancer, but when you give them together, the effect is what I’d call one plus one equals three.”
The team saw something similar in study participants. When they gave them only tucatinib, some people’s tumors stopped growing for a while but none shrank, and they ended up having trastuzumab added to their treatment, he explained.
Long-lasting tumor control for some people
Seagen Inc., which manufactures tucatinib, funded the team to conduct the larger MOUNTAINEER trial, which tested the combination of tucatinib and trastuzumab in 84 study participants whose advanced colorectal cancer had come back or hadn’t shrunk after previous treatments. Most participants had metastatic tumors in their liver and lungs.
Participants also had to have genetic tests showing that their cancer didn’t have RAS mutations. Such mutations are thought to allow cancer cells to keep growing even if HER2 is blocked, rendering that treatment useless, explained Dr. Strickler.
MOUNTAINEER participants received tucatinib and trastuzumab until their cancer started growing again, or until they experienced side effects.
At the time the trial results were presented at the European Society for Medical Oncology World Congress on Gastrointestinal Cancers in 2022, participants’ tumors had continued to respond to the treatment for a median of just over a year.
It will be important to keep following the remaining patients in the MOUNTAINEER trial for a while, said Dr. Allegra. “For those patients who did get a response, it lasted a long time, relatively speaking,” he said. “But whether that response rate will translate into a survival advantage over other treatments isn’t known yet.”
Median overall survival in the MOUNTAINEER trial was just over 2 years. In large clinical trials, said Dr. Allegra, overall survival has been about 7 months for people with advanced colorectal cancer who have already had several treatments. However, those whose tumors overexpress HER2 and don’t have RAS mutations may tend to live longer regardless of what treatment they receive, he added.
The most common side effects seen during the MOUNTAINEER trial were diarrhea, fatigue, rash, nausea, fever, and reactions to the trastuzumab infusions such as chills. The most common serious side effect was high blood pressure. Doses of tucatinib were reduced or stopped for 6% of the 84 participants due to side effects.
However, the severity and frequency of side effects with the targeted drug combination was less than that seen with the chemotherapy drugs that would normally be used for these patients, said Dr. Strickler.
Combination therapies for longer responses?
These results of the MOUNTAINEER trial led to an ongoing follow-up study called MOUNTAINEER-03. This randomized clinical trial is testing the addition of tucatinib and trastuzumab to standard treatment regimens using chemotherapy and other targeted therapies, as initial treatment for metastatic HER2-positive colorectal cancer.
Other trials are also testing combinations of other targeted drugs in patients with previously treated advanced colorectal cancer to see if they, like tucatinib and trastuzumab, work better when given together.
For example, explained Dr. Allegra, another phase 3 clinical trial recently tested the combination of bevacizumab (Avastin) with trifluridine and tipiracil (Lonsurf) in people with advanced colorectal cancer who had received at least two previous treatments. Those participants lived for a median of almost a year after starting treatment. Participants in that trial could receive the study drug combination regardless of the gene mutations found in their tumors.
For people with advanced HER2-positive tumors, researchers are interested in testing other treatments that target the protein, such as trastuzumab deruxtecan (Enhertu), which is a type of drug called an antibody-drug conjugate, Dr. Strickler explained.
If these could be used after a recurrence to delay chemotherapy even further for these patients, “that could give them more options and better quality of life,” he said.