FDA Approves Trastuzumab Deruxtecan for Any HER2-Positive Solid Cancer
, by Carmen Phillips
The drug trastuzumab deruxtecan (Enhertu) can now be used to treat a wide variety of cancers, thanks to a new approval from the Food and Drug Administration (FDA).
On August 5, the agency gave an accelerated approval for trastuzumab deruxtecan—often called T-DXd—to treat anyone with any advanced solid cancer if their tumors produce high levels of the protein HER2, or HER2-positive. To receive the drug, a patient must already have received at least one prior treatment.
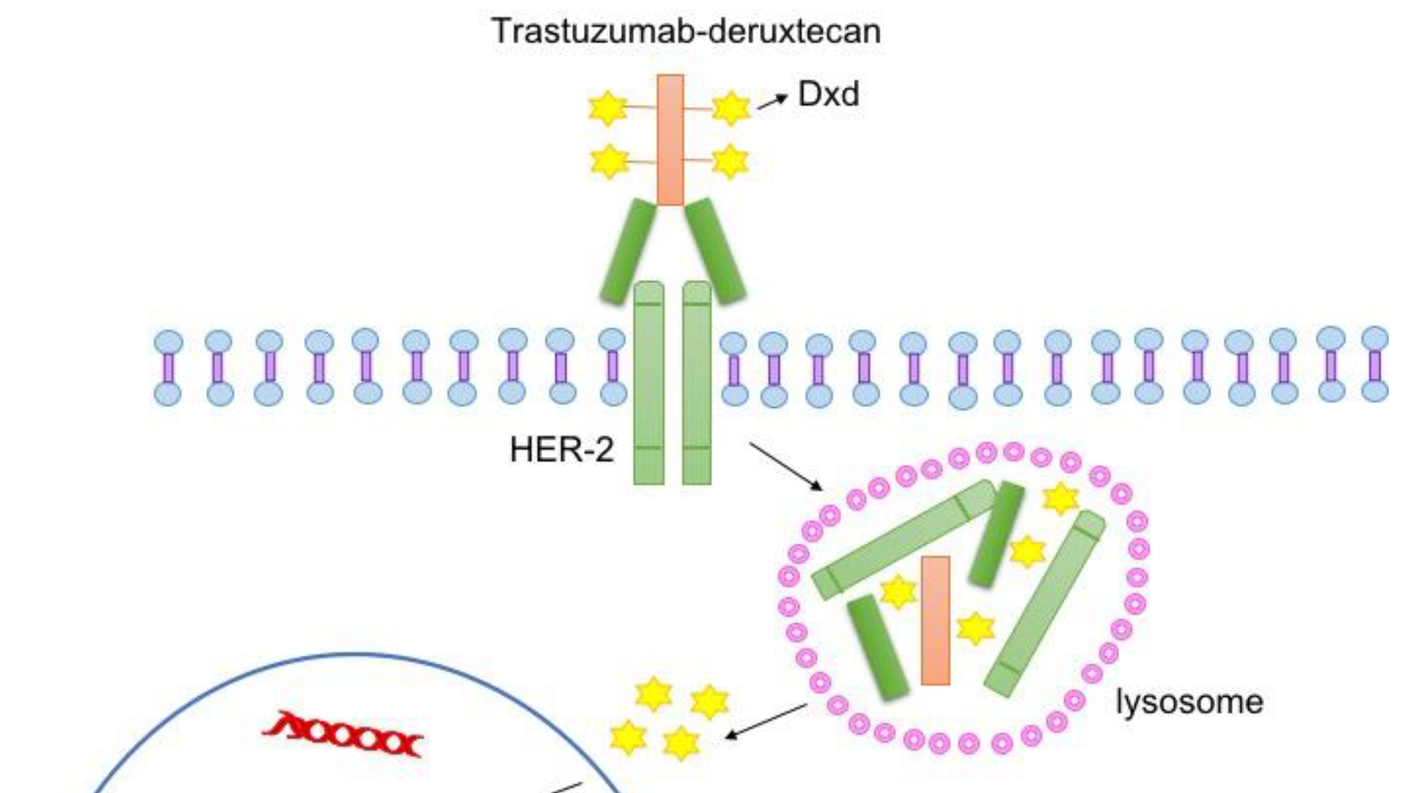
The approval makes T-DXd the first cancer therapy of its type, an antibody─drug conjugate, that can be used in such a “tumor agnostic” manner. T-DXd is already approved to treat people with several specific cancers, including breast and stomach.
The new approval was based on results from three midsized clinical trials in which treatment with T-DXd shrank tumors (a tumor response) in many people with advanced HER2-positive cancers. In many instances, their tumors did not start growing again for many months, or even several years.
In its approval, FDA highlighted that T-DXd can have serious side effects, including a dangerous lung inflammation called interstitial lung disease (ILD). In the trials, some patients died from ILD.
Oncologists will have to consider T-DXd’s potential side effects when evaluating whether to recommend it to their patients, said Funda Meric-Bernstam, M.D., of the University of Texas MD Anderson Cancer Center and the lead investigator on one of the three trials.
But there’s now substantial evidence that it has “compelling activity” in many different types of cancer, Dr. Meric-Bernstam continued. Based on that evidence, she said, “I expect there will be substantial uptake [of T-DXd]” among oncologists.
From breast cancer to clinical trials in a host of cancers
Trastuzumab (Herceptin), a type of drug known as a monoclonal antibody, was one of the first FDA-approved targeted therapies for cancer. It works by seeking out and latching on to HER2 proteins on cancer cells, preventing the protein from helping tumors grow and encouraging immune cells to attack the cells.
In the case of T-DXd, trastuzumab has a partner to which it’s chemically linked, the chemotherapy drug deruxtecan. Trastuzumab’s job in T-DXd is to be the delivery driver, routing the drug to its final address: cells with HER2 on their surface. After it latches on to HER2, the entire drug is pulled into the cell and the cargo, deruxtecan, is unleashed to kill the cell.
Trastuzumab was developed in the 1990s specifically to treat breast cancer. That’s because studies at the time had shown that tumors in about 20% of people with breast cancer had an overabundance of HER2 on their surfaces, known as HER2 overexpression.
Over the past 10–15 years, however, scientists have repeatedly found that this HER2 overload occurs in many other cancers. During that same time, FDA has approved multiple drugs that target HER2, including T-DXd.
With convincing data and multiple treatments available to them, many clinical trials were launched to test the different HER2-targeted therapies in people with cancers in which HER2 overexpression is common. Among them were the three phase 2 trials of T-DXd—funded by AstraZeneca and Daiichi Sankyo, the drug’s manufacturer—on which this new FDA approval was based.
Strong and often long-lasting responses to T-DXd in many cancer types
Of the three trials, one enrolled only people with advanced lung cancer, one only people with advanced colorectal cancer, and the third people with a variety of advanced solid cancers.
Participants in all three trials had HER2-positive tumors. However, while most participants’ tumors had the highest HER2 levels, a measure known as IHC3+, others had somewhat lower levels, known as IHC2+.
In the “pan-tumor” trial, more than half of people with IHC3+ tumors responded to the treatment. Many of those tumor responses lasted for 20 months or more, although some were limited to a few months.
The most impressive responses were seen in people with IHC3+ gynecologic cancers. For example, about 85% of people with endometrial cancer and 75% of people with cervical cancer responded to T-DXd. Many of these responses lasted for at least a year, and some lasted much longer.
Response rates were also high in participants with rare, hard-to-treat cancers. More than 56% of those with IHC3+ biliary tract cancer, for example, had a response, some lasted for nearly 2 years.
And 42% of people with salivary gland cancer had a tumor response, including several that lasted beyond 20 months.
In the lung and colorectal cancer trials, tumors also shrank in about half of patients. The length of those responses, however, were generally much shorter than was seen for other tumor types.
Being proactive about ILD
T-DXd’s most common side effects include nausea, anemia, and fatigue. In the pan-tumor trial, about 30% of participants had their dose of the drug lowered or stopped treatment altogether because of side effects.
Overall, about 10% to 15% of people treated with T-DXd develop ILD, the most common form of which is called pneumonitis.
“Not all oncologists may be familiar with interstitial lung disease or pneumonitis, [and it’s] an important adverse event to watch out for,” Dr. Meric-Bernstam said.
In many cases, when ILD does develop, treating it is straightforward. But she cautioned that it’s important for oncologists to take steps to catch it early and manage it effectively.
New option where new options are desperately needed
For a difficult-to-treat cancer like salivary gland cancer, having an effective new treatment for many patients is a major advance, said Ezra Cohen, M.D., who specializes in treating the disease at the University of California San Diego Moores Cancer Center.
Only about 2,400 people are diagnosed with salivary gland cancer in the United States each year. There are many subtypes of salivary gland cancer, but in the one of the most aggressive ones, salivary ductal carcinoma, about 40% have HER2-positive tumors, Dr. Cohen noted.
“There is no standard first-line treatment” for advanced salivary gland cancer, he explained. With this new approval, he said, using T-DXd as an initial, or first-line, treatment for patients with HER2-positive disease rather than as a second-line treatment is now a consideration.
Another small trial showed strong responses of salivary gland tumors to a different HER2-targeted drug, ado-trastuzumab emtansine (Kadcyla). Based on the results from that and the T-DXd trial, he and other oncologists have been using both drugs to treat some people with the disease, Dr. Cohen noted.
“My experience [with them] thus far … has been extraordinarily positive,” he said.
Dr. Meric-Bernstam called the strong and long-lasting tumor responses in people with gynecologic cancers “very exciting.” Based on the results from the pan-tumor trial, she added, T-DXd has already been included in widely used guidelines as a potential treatment option for HER2-positive gynecologic cancers.
Having this new FDA approval, both researchers noted, will help ensure that insurance covers the treatment.
“The mandate now,” Dr. Cohen stressed, “is we have to test all of our patients [for HER2 overexpression] because we have effective therapies” for them.