Treatment - Cancer Currents Blog
Cancer treatment related news, with context from leading experts. Includes articles on new therapies, treatment side effects, and important trends in treatment-related research.
-
Durvalumab Extends Lives of People with Early-Stage Small Cell Lung Cancer
The immunotherapy drug durvalumab (Imfinzi) can help people with early-stage small cell lung cancer live longer, results from a large clinical trial show. Three years after starting treatment, nearly 60% of people who received the drug were still alive.
-
Spurred by Survivors, Researchers Are Revisiting Cancer Drug Doses
When it comes to cancer drugs, researchers are moving away from a paradigm called the maximum tolerated dose. Instead, they’re focusing more on identifying doses that produce fewer side effects but are still effective against a person’s cancer.
-
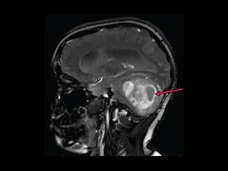
Tovorafenib Approved for Some Children with Low-Grade Glioma
FDA has granted an accelerated approval to tovorafenib (Ojemda) for kids and teens who have low-grade glioma with changes in the BRAF gene. In a small clinical trial, the drug shrank or completely eliminated tumors in nearly 70% of patients.
-
Pembrolizumab Is First Adjuvant Therapy to Improve Overall Survival in Kidney Cancer
In a large clinical trial, treatment with pembrolizumab after surgery helped people with kidney cancer live longer than those who got a placebo and standard monitoring. The findings mark the first time an adjuvant treatment for kidney cancer has improved survival.
-
Alectinib Approved as an Adjuvant Treatment for Lung Cancer
FDA has approved alectinib (Alecensa) as adjuvant therapy for people with lung cancer who have ALK-positive tumors. In a clinical trial, alectinib helped people live longer after surgery without their cancer returning than chemotherapy.
-
FDA Approves Trastuzumab Deruxtecan for Any HER2-Positive Solid Cancer
Trastuzumab deruxtecan (Enhertu) can now be used to treat any advanced solid cancer that produces high levels of the protein HER2 (HER2-positive tumors). FDA’s accelerated approval was based on findings from three clinical trials.
-
Approval of Elahere Expands Treatment Options for Some Advanced Ovarian Cancers
FDA approved mirvetuximab soravtansine-gynx (Elahere) to treat people with advanced, platinum-resistant ovarian cancer whose tumors overproduce a protein called FR-α. The full approval was based on the results of a large, randomized trial called MIRASOL, which showed Elahere improved survival for these people.
-
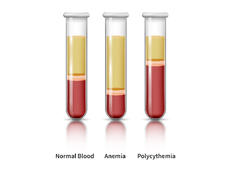
Rusfertide Nearly Eliminates Need for Phlebotomies to Treat Polycythemia Vera
Adding rusfertide to treatment for polycythemia vera cut phlebotomy rates from nine to less than one per year in a recent clinical trial. The finding may improve the quality of life for those who manage the blood cancer with frequent blood draws.
-
Simple Hysterectomy May Expand Treatment Options for Early-Stage Cervical Cancer
For some people with early-stage cervical cancer, a surgical procedure called a simple hysterectomy may be a safe and effective alternative to treatment with a radical hysterectomy, results from the SHAPE trial show.
-
Nivolumab Injections Could Make Treatment Easier for More People with Cancer
In a clinical trial, an injectable form of nivolumab (Opdivo) was as effective against kidney cancer as the intravenous form of the drug. Side effects were also similar and treatment time was shorter. Injectable immunotherapies, several experts said, if found to be comparable to IV forms, can be more convenient to receive and accessible to more people.
-
First Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma
In an event more than three decades in the making, FDA has approved lifileucel (Amtagvi), the first cancer treatment that uses immune cells called tumor-infiltrating lymphocytes, or TILs.
-
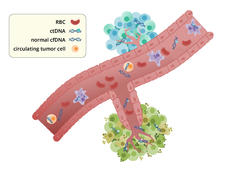
ctDNA May Guide Who Needs Chemo After Colorectal Cancer Surgery
Results from a new study suggest that the presence of circulating tumor DNA (ctDNA) in blood samples can predict which patients with colorectal cancer should and shouldn’t get chemotherapy after surgery to remove their tumors.
-
Repotrectinib Expands Treatment Options for Lung Cancers with ROS1 Fusions
The results of the clinical trial that led to FDA’s 2023 approval of repotrectinib (Augtyro) for lung cancers with ROS1 fusions have been published. The drug shrank tumors in 80% of people receiving the drug as an initial treatment.
-
India’s First Homegrown CAR T-Cell Therapy Has Roots in NCI Collaboration
Training provided by NCI scientists helped researchers in India design an effective CAR-T cell therapy, NexCAR19, that can be manufactured in India, made available at a reasonable cost, and meet the needs of patients in India’s health care system.
-
Can Some People with Breast Cancer Safely Skip Lymph Node Radiation?
Some people with no evidence of cancer in nearby lymph nodes after presurgical chemotherapy can skip radiation to that area without increasing the risk of the cancer returning, a clinical trial found. But some experts caution that more details are needed.
-
Trial Results Support Adding Daratumumab to Initial Treatment for Multiple Myeloma
Adding daratumumab (Darzalex) to standard treatment helped people with newly diagnosed multiple myeloma live longer without their cancer getting worse or dying. People taking daratumumab were also more likely to have no detectable signs of cancer (minimal residual disease) after treatment.
-
Enzalutamide Gets Added Approval for Prostate Cancer That Hasn’t Spread
Under a new FDA approval, enzalutamide (Xtandi) can now be used alone, or in combination with leuprolide, to treat people with nonmetastatic prostate cancer that is at high risk of returning after surgery or radiation.
-
Toripalimab Becomes First Immunotherapy Drug Approved for Nasopharyngeal Cancer
FDA approved toripalimab (Loqtorzi) based on the results of a large clinical trial showing that, when added to chemotherapy, the drug extended survival in people with nasopharyngeal cancer that returned after initial treatment or spread in the body.
-
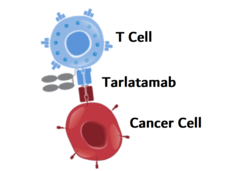
Tarlatamab Shows Promise for Some People with Small Cell Lung Cancer
Tarlatamab, a new type of targeted immunotherapy, shrank small cell lung cancer (SCLC) tumors in more than 30% of participants in an early-stage clinical trial. Participants had SCLC that had progressed after previous treatments with other drugs.
-
Groundbreaking Trial Results Expand Treatment Options for Some People with Bladder Cancer
For the first time in decades, people with advanced bladder cancer have more effective treatment options. New clinical trial results mark a pivotal moment following years of little progress, bladder cancer experts believe.