Cancer Currents: An NCI Cancer Research Blog
A blog featuring news and research updates from the National Cancer Institute. Learn more about Cancer Currents.
-
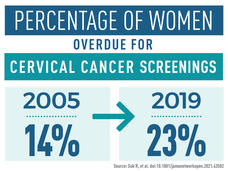
Why Are Many Women Overdue for Cervical Cancer Screening?
The rates of timely cervical cancer screening fell between 2005 and 2019, researchers found, and disparities existed among groups of women. The most common reason for not receiving timely screening was lack of knowledge about screening or not knowing they needed screening.
-
Can Chronic Graft-Versus-Host Disease Be Prevented?
Removing immune cells called naive T cells from donated stem cells before they are transplanted may prevent chronic graft-versus-host disease (GVHD) in people with leukemia, a new study reports. The procedure did not appear to increase the likelihood of patients’ cancer returning.
-
Durvalumab Modestly Improves Survival in Advanced Biliary Tract Cancer
Adding durvalumab (Imfinzi) to standard chemotherapy modestly extended how long people with advanced biliary tract cancer lived, results from the TOPAZ-1 trial show. The immunotherapy drug may now be the standard first-line therapy for this hard-to-treat cancer.
-
Air Is Life: The Navajo Nation’s Historic Commercial Tobacco Ban
With the passage of the Air is Life Act of 2021, the Navajo Nation enacted the most comprehensive ban on commercial tobacco products of any American Indian tribe to date.
-
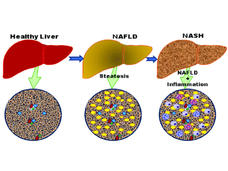
Study Identifies Potential Drug Target to Prevent Some Liver Cancers
Researchers have found that mice that lack β2-spectrin protein in their livers are protected from nonalcoholic fatty liver disease and the most common kind of liver cancer, hepatocellular carcinoma.
-
President’s Cancer Panel Report: Closing Gaps in Cancer Screening for All Americans
Dr. John P. Williams, chair of the President’s Cancer Panel, summarizes the panel’s new report, Closing Gaps in Cancer Screening: Connecting People, Communities, and Systems to Improve Equity and Access.
-
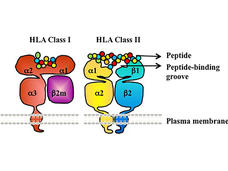
Cancer Immunotherapies Don’t Work for Everyone: HLA Gene May Explain Why
A specific form of the HLA gene, HLA-A*03, may make immune checkpoint inhibitors less effective for some people with cancer, according to an NCI-led study. If additional studies confirm the finding, it could help guide the use of these commonly used drugs.
-
Even If Insured, People with Advanced Cancer Often Face Financial Problems
Financial hardship caused by cancer care was common and occurred early in treatment, even for patients with insurance, a recent study found. The investigators believe financial hardship should be addressed like any other complication of cancer care.
-
Can mRNA Vaccines Help Treat Cancer?
The success of mRNA vaccines for COVID-19 could help accelerate research on using mRNA vaccine technology to treat cancer, including the development of personalized cancer vaccines.
-
Should CAR T Cells Be Used Earlier in People with Non-Hodgkin Lymphoma?
Results from three clinical trials have found that CAR T cells may be superior to standard treatments for patients with B-cell NHL that has not responded to treatment (refractory) or has returned after treatment (relapsed).
-
When Ovarian Cancer Returns, Surgery May Be a Good Choice for Selected Patients
For patients with recurrent ovarian cancer who meet strict criteria, additional surgery may improve survival, results from a large clinical trial show.
-
FDA Oversight of E-Cigarettes Gathers Speed: A Conversation with Mitch Zeller
Companies that want to market e-cigarettes in the United States must now submit applications to the Food and Drug Administration (FDA). In this interview, Mitch Zeller, director of FDA’s Center for Tobacco Products, provides insights into FDA actions on e-cigarettes.
-
Black Patients Are More Likely to Die of Cancer—Here’s How One Group Is Tackling That
A program called ACCURE is showing promise toward reducing disparities among Black and White patients with breast and lung cancers. ACCURE involves system-wide changes at cancer centers to overcome structural and cultural barriers in patient care.
-
Immunotherapy Combination Most Effective as Initial Treatment for BRAF+ Melanoma
Clinical trial finds that ipilimumab (Yervoy) and nivolumab (Opdivo) combo is superior to a combination of the targeted therapies dabrafenib (Tafinlar) and trametinib (Mekinist) as the first treatment for metastatic BRAF-positive melanoma.
-
For Older Adults, Geriatric Assessment Reduces Cancer Treatment Side Effects
For older adults with advanced cancer, a geriatric assessment can help direct their treatment, a new study shows. In the study, patients whose care was guided by a geriatric assessment experienced fewer serious side effects and falls in their homes.
-
Tumors May Shed Protein to Create Barriers that Block Immune Cells
Cancer cells can shed a protein called DDR1 that helps collagen proteins create dense barriers around tumors. A study in mice showed these barriers can prevent immune cells from entering and killing tumors.
-
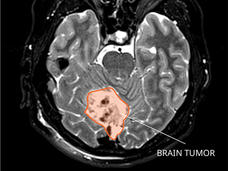
Test Detects Early Signs of Remaining Cancer in Kids Treated for Medulloblastoma
A new test could potentially be used to identify children treated for medulloblastoma who are at high risk of their cancer returning. The test detects evidence of remaining cancer in DNA shed from medulloblastoma tumor cells into cerebrospinal fluid.
-
New Risk Model Aims to Reduce Breast Cancer Disparities in Black Women
Most breast cancer risk tools were developed with data mainly from White women and don’t work as well for Black women. A new tool that estimates risk for Black women may help identify those who might benefit from earlier screening, enabling earlier diagnosis and treatment.
-
Shorter, More Intensive Radiation Safe after Surgery for Prostate Cancer
Many with prostate cancer can safely receive shorter, higher-dose radiation therapy after surgery, a new study has found. The approach, called HYPORT, didn’t harm patients’ quality of life compared with the standard radiation approach, trial finds.
-
Tecartus Becomes First CAR T-Cell Therapy Approved for Adults with ALL
The CAR T-cell therapy Tecartus has become the first such treatment approved by FDA to treat adults with acute lymphoblastic leukemia (ALL). The approval is for patients whose cancer has not responded to treatment or returned after treatment.