May 2024 - Cancer Currents Blog
-
Leading Change in Cancer Clinical Research, Because Our Patients Can’t Wait
Reshaping the cancer clinical trials infrastructure to overcome key bottlenecks will involve embracing technology and collaboration, and inviting innovation, explain NCI Director Dr. W. Kimryn Rathmell and NCI Special Advisor Dr. Shaalan Beg.
-
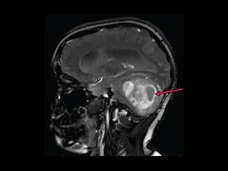
Tovorafenib Approved for Some Children with Low-Grade Glioma
FDA has granted an accelerated approval to tovorafenib (Ojemda) for kids and teens who have low-grade glioma with changes in the BRAF gene. In a small clinical trial, the drug shrank or completely eliminated tumors in nearly 70% of patients.
-
Pembrolizumab Is First Adjuvant Therapy to Improve Overall Survival in Kidney Cancer
In a large clinical trial, treatment with pembrolizumab after surgery helped people with kidney cancer live longer than those who got a placebo and standard monitoring. The findings mark the first time an adjuvant treatment for kidney cancer has improved survival.
-
New Approach May Help People with Cancer Better Manage Depression, Pain, and Fatigue
Assessing and offering people with cancer stepped collaborative care may help better manage symptoms of depression, pain, and fatigue than the standard referral to providers for treatment, according to a recent study.
-
Blood Test Accurately Detects Early-Stage Pancreatic Cancer
In a new study involving nearly 1,000 people, a liquid biopsy accurately detected early- and late-stage pancreatic cancer. When paired with a test for the protein CA19-9, the combination accurately identified 97% of people with early-stage disease.
-
Alectinib Approved as an Adjuvant Treatment for Lung Cancer
FDA has approved alectinib (Alecensa) as adjuvant therapy for people with lung cancer who have ALK-positive tumors. In a clinical trial, alectinib helped people live longer after surgery without their cancer returning than chemotherapy.
-
FDA Approves Trastuzumab Deruxtecan for Any HER2-Positive Solid Cancer
Trastuzumab deruxtecan (Enhertu) can now be used to treat any advanced solid cancer that produces high levels of the protein HER2 (HER2-positive tumors). FDA’s accelerated approval was based on findings from three clinical trials.
-
Q&A: Learning About the Cancer Care Challenges LGBTQ+ People Face
Gwendolyn Quinn, Ph.D., a health psychologist at NYU Langone Health Perlmutter Cancer Center, discusses the challenges faced by sexual and gender minority people being treated for cancer and ways to address them.
-
LGBTQ+ Voices: Listening to Sexual and Gender Minority People Affected by Cancer
LGBTQ+ people, also known as sexual and gender minorities (SGM), with cancer may face challenges that non-SGM people do not face. The breadth and depth of these disparities aren't well known, but there’s growing research on the challenges this diverse group faces.