Revumenib Shows Promise in Treating Advanced Acute Myeloid Leukemia
, by Sharon Reynolds
A new type of targeted therapy shows promise for acute myeloid leukemia (AML), one of the most difficult leukemias to treat. The drug, revumenib, is part of a group, or class, of drugs known as menin inhibitors.
In an early-phase clinical trial called AUGMENT-101, treatment with revumenib caused about one-third of study participants’ cancers to completely disappear, known as a complete remission. All of the participants had previously received many other treatments, including in some cases a stem cell transplant.
The trial didn’t directly compare revumenib with any other drugs. But historically, “if patients’ cancers have progressed on that many lines of therapy, the chances of responding to anything else we [currently] have is less than 10%,” said Ghayas Issa, M.D., of the University of Texas MD Anderson Cancer Center, who helped lead the trial.
According to the most recent data from the study, published on March 15 in Nature, one participant was still in remission more than 16 months after starting treatment, and 12 more who had gone into remission went on to receive a stem cell transplant. Of this group, 9 continued to be in remission at the time of the last analysis, Dr. Issa and his colleagues reported.
Very few patients with recurrent leukemia can achieve remission—and stay healthy enough to receive a transplant—with current standard therapies, said Dr. Eytan Stein, M.D., of Memorial Sloan Kettering Cancer Center, who also helped lead the trial.
Several other menin inhibitors are also being tested in early-phase trials, said Eunice Wang, M.D., of Roswell Park Comprehensive Cancer Center, who was not involved in the study. “This could be the beginning of a new class of agents [for treating AML], which … is causing a lot of excitement,” she added.
Removing the key that starts the leukemia engine
Many new treatments that have been developed, such as ivosidenib (Tibsovo) and midostaurin (Rydapt), target genetic changes thought to drive some forms of AML. But resistance to these drugs tends to develop relatively quickly. And each of the gene changes targeted by these drugs are found in only a minority of patients.
“About 50% to 60% of acute myeloid leukemias don’t have any of those mutations. And that leaves us with just toxic chemotherapy [for treatment],” said Dr. Wang.
However, many AMLs do have one of two other common gene changes, KMT2A rearrangements and NPM1 mutations. These mutations cause blood cells to regress, or dedifferentiate, and behave like the stem cells they arose from. The result is the formation of leukemia cells instead of functional blood cells.
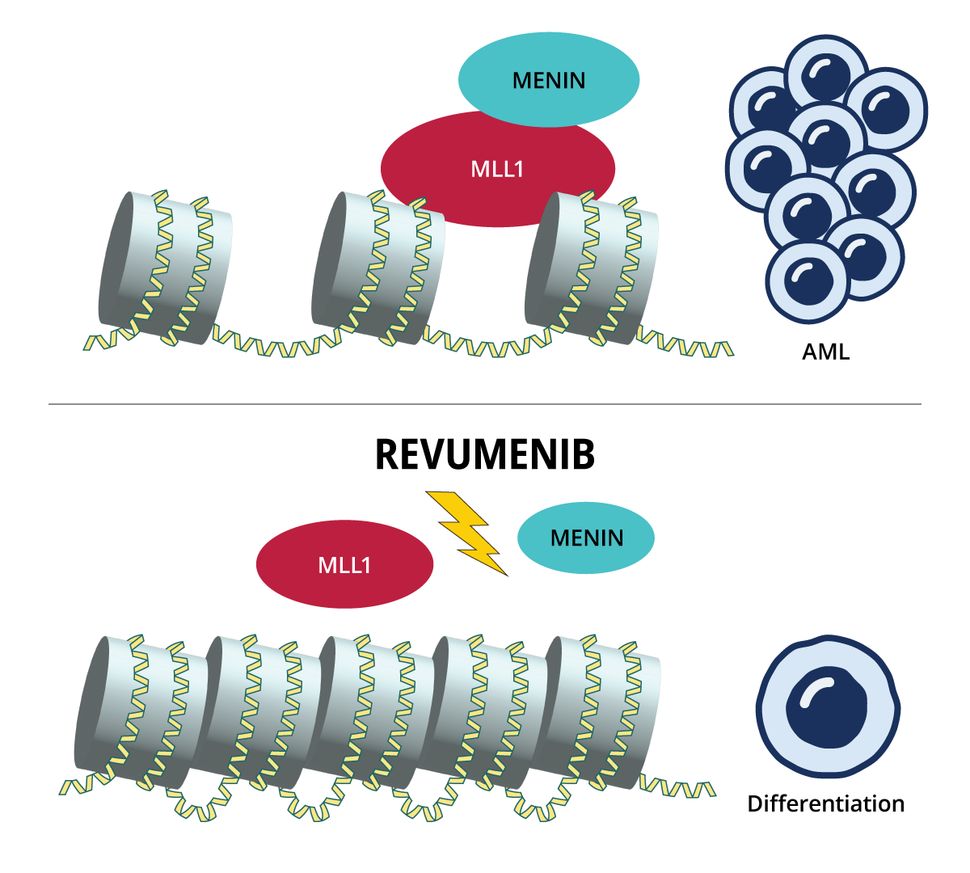
The cellular programs hijacked by both these gene changes require menin, which binds to the protein produced by KMT2A, called MLL1. The complex formed by these proteins in turn binds to chromatin (a tightly packed collection of DNA and proteins within the cell’s nucleus) and turns on the aberrant communication pathways triggered by the altered KMT2A or NPM1.
Revumenib and other menin inhibitors work differently from other targeted therapies used in AML to date. Instead of blocking the activity of dysfunctional proteins, menin inhibitors stop the genes affected by the altered KMT2A or NPM1 from being expressed in the first place.
Dr. Issa describes the menin-MLL1 complex as the key that starts the engine of leukemia cell production.
“And [revumenib] creates a wedge between the key and the engine,” by binding to menin, which prevents it from binding to MLL1, he explained. When the menin-MLL1 complex can’t bind to chromatin, the cells that had acted like haywire stem cells either turn back into normal cells (a process called differentiation) or die.
What’s exciting about revumenib, Dr. Stein said, is not just the novel mechanism by which menin inhibition works, but that cancer-causing mutations that rely on menin are very common in AML.
“All told, menin inhibition may have a role in potentially 40% to 50% of all acute myeloid leukemias,” he said.
Pushing blood cells to behave
The phase 1 AUGMENT-101 trial—which was funded by Syndax Pharmaceuticals, the maker of revumenib—was the first to test the new drug in people. It was designed to test the drug’s safety and find the best dose for future, larger trials.
In total, 60 adults and 8 children with leukemia—most with AML—joined the trial. Participants had received a median of four prior treatments, and almost half had already undergone a stem cell transplant.
Because this was a phase 1 clinical trial, researchers were trying to determine the best dose of the drug to use in larger trials. To do so, the first small groups of participants took a specific dose and, if no serious side effects developed, the next group of patients received a higher dose. This process played out until a dose was found that led to unacceptable side effects—known as the maximum tolerated dose.
Trial participants took revumenib as a pill or, if unable to take pills, in liquid form twice a day, in escalating doses [see box], until their leukemia started growing again or they experienced unacceptable side effects.
Overall, 18 of the 60 patients who had a KMT2A rearrangement or NPM1 mutation experienced a complete remission with a full or partial recovery of the number of healthy blood cells, which lasted for a median of 9 months.
Twelve of these participants received a stem cell transplant after going into remission.
“Nearly all patients with [recurrent AML] are unable to get into remission with standard chemotherapy, and a successful transplant requires a patient to be in remission,” Dr. Stein said.
The side effect seen in the trial that helped the researchers identify the maximum tolerated dose for future studies was called QT interval prolongation, which is a problem with the heart’s electrical activity. Other serious side effects seen during treatment included a drop in the number of white and red blood cells. No participants had to stop taking revumenib permanently due to side effects, and no deaths were caused by the drug.
A potentially dangerous side effect of some leukemia treatments is called differentiation syndrome. This is caused by an immune-system reaction triggered when leukemia cells start to change back into normal cells. Although 11 patients developed differentiation syndrome, none of the cases were severe, and all disappeared with anti-inflammatory treatments.
During treatment, the trial investigators tracked gene expression changes in bone marrow cells taken from participants. As expected, they saw less activity of genes that drive leukemia and more activity of genes associated with healthy blood cell differentiation.
Tracking treatment resistance in real time
For some participants in AUGMENT-101, their cancers rapidly developed resistance to revumenib. In a companion study published in the same issue of Nature, a team led by Sheng Cai, M.D., Ph.D., and Ross Levine, M.D., of Memorial Sloan Kettering, and Scott Armstrong, M.D., Ph.D., of Dana-Farber Cancer Institute, teased out exactly what changes in leukemia cells were driving this resistance.
The team used multiple methods as part of this work, including x-ray crystallography to view the physical interactions of revumenib with the menin-MLL protein complex as well as whole genome sequencing.
Some patients whose AML initially responded to menin but then started growing again had new changes in the MEN1 gene, which encodes menin. Selection for these rare or new mutations happened as early as 2 months after starting treatment. In follow-up laboratory studies, the researchers saw that, without revumenib, cells with these new MEN1 changes were actually less likely to survive and thrive.
But that changed quickly once revumenib was added. The changes to MEN1 made it so revumenib could no longer disrupt menin from binding to MLL1. “So, the leukemia genes continue to be turned on, and that’s the basis of the resistance,” Dr. Cai said.
Although developing resistance presents a challenge, he added, it also confirmed the importance of menin to these types of AML. “The fact that [leukemia cells] have to mutate in order for them to escape this drug really means that we’re getting at the Achilles' heel of these leukemia subtypes,” he said.
Inhibiting menin with combination therapy, and earlier
The first generation of menin inhibitors shows promise in early trials. But Dr. Cai hopes that the structural information his team gathered may inform future drug development. “Seeing the way that the drug interacts with the protein gives us a lens to see where, strategically, we might be able to design newer menin inhibitors that work in other ways,” he said.
Revumenib is now being tested in a phase 2 study, with the goal of obtaining FDA approval for treating people with advanced AML with these specific gene alterations, Dr. Stein said. “And we have ongoing studies looking at combination therapies,” he added. For example, his team is now testing revumenib with venetoclax (Venclexta), another targeted therapy that has shown promise against AML.
Other potential strategies include combining menin inhibitors with standard chemotherapy, said Dr. Wang. “That way, maybe we could kill some of these [cells] that are becoming resistant by some other mechanism,” she said. The ongoing phase 1 AUGMENT-102 trial is looking at this type of combination in people whose leukemia has relapsed or not responded to at least two prior treatments.
Combinations of treatments that include a menin inhibitor may work best immediately after a diagnosis of leukemia, Dr. Wang added.
“To harvest the true benefit of [menin inhibitors], we may really need to move them up earlier in treatment, when we have the best chance at a cure,” she said.