Radiopharmaceuticals: Radiation Therapy Enters the Molecular Age
, by NCI Staff
The past two decades have brought a sea change in the way many types of cancer are treated. Targeted therapies shut down specific proteins in cancer cells that help them grow, divide, and spread. Immunotherapies stimulate or suppress the body’s immune system to help fight cancer. But long-used treatments — surgery, chemotherapy, and radiation therapy — remain the backbone of treatment for most cancers.
Radiation therapy was first used to treat cancer more than 100 years ago. About half of all cancer patients still receive it at some point during their treatment. And until recently, most radiation therapy was given much as it was 100 years ago, by delivering beams of radiation from outside the body to kill tumors inside the body.
Though effective, external radiation can also cause collateral damage. Even with modern radiation therapy equipment, “you have to [hit] normal tissue to get to a tumor,” said Charles Kunos, M.D., Ph.D., of NCI’s Cancer Therapy Evaluation Program (CTEP). The resulting side effects of radiation therapy depend on the area of the body treated but can include loss of taste, skin changes, hair loss, diarrhea, and sexual problems.
Now, researchers are developing a new class of drugs called radiopharmaceuticals, which deliver radiation therapy directly and specifically to cancer cells. The last several years have seen an explosion of research and clinical trials testing new radiopharmaceuticals.
These studies have suggested that targeting radiation therapy at the cellular level has the potential to reduce the risk of both short- and long-term side effects of treatment while at the same time enabling even tiny deposits of cancer cells to be killed throughout the body.
“I think they’re going to transform radiation oncology in the next 10 to 15 years,” Dr. Kunos said.
Building on a Natural Affinity
Delivering radiation directly to cells isn’t itself a new approach. One such therapy, called radioactive iodine, has been used to treat some types of thyroid cancer since the 1940s. Iodine naturally accumulates in thyroid cells. A radioactive version of the element can be produced in the lab. When ingested (as a pill or a liquid), it accumulates in and kills cancer cells left over after thyroid surgery.
A similar natural affinity was later exploited to develop drugs to treat cancer that has spread to the bones, such as radium 223 dichloride (Xofigo), which was approved in 2013 to treat metastatic prostate cancer. When cancer cells grow in the bone, they cause the bone tissue they invade to break down. The body then attempts to repair this damage by replacing that bone—a process called bone turnover.
The radioactive element radium “looks like a calcium molecule, so it gets incorporated into areas of the body where bone turnover is highest,” such as areas where cancer is growing, Dr. Kunos explained. The radium is then able to kill nearby cancer cells.
These radioactive compounds all travel to cancer cells without any help. Researchers wondered whether it would be possible to engineer new radioactive molecules that specifically target other cancers.
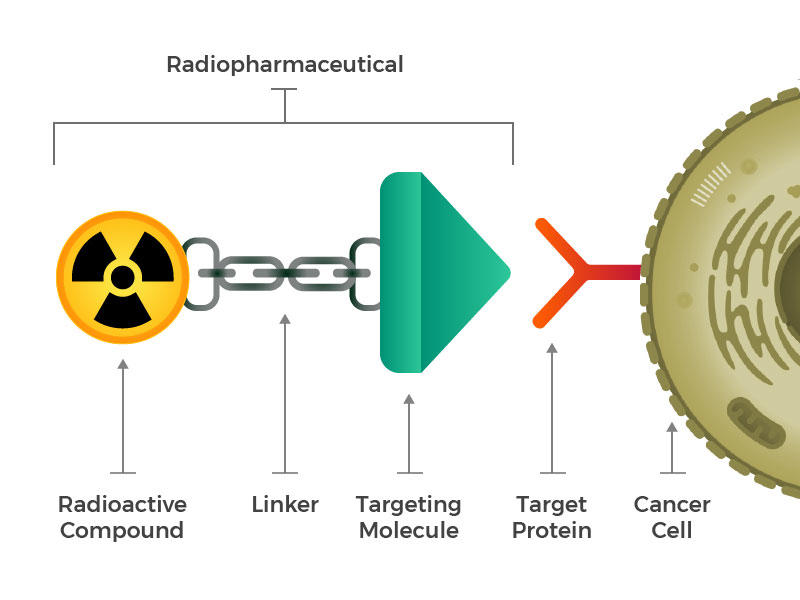
They envisioned engineered radiopharmaceuticals that consist of three main building blocks: a radioactive molecule, a targeting molecule (that recognizes and latches specifically onto cancer cells), and a linker that joins the two. Such compounds could be injected, infused, inhaled, or ingested, and then make their way into the bloodstream.
The idea of linking a cancer-targeting molecule with a molecule that kills cancer cells is not new either. For example, several drugs called antibody–drug conjugates, in which an antibody that binds to specific cancer cells is linked to a toxic drug, have been approved for treating cancer.
But efforts to create such drugs have met with limited success, Dr. Kunos explained, because it isn’t enough that toxins be brought close to a cancer cell. The toxins have to be taken inside and stay inside the cells long enough to cause damage. Many cancer cells have or develop mechanisms to simply pump toxins right back out before that can happen.
Radiopharmaceuticals also work best when the drugs can get inside cells. But that’s not necessary for them to be effective. Once a radiopharmaceutical has stuck to a cancer cell, the radioactive compound naturally breaks down. This decay releases energy that damages the DNA of nearby cells. And when a cell’s DNA is irreparably damaged, that cell dies. Cancer cells are particularly sensitive to radiation-induced DNA damage.
Depending on the type of radioactive compound used, the resulting energy can penetrate the cell bound to the radiopharmaceutical as well as about 10 to 30 cells surrounding that cell. This increases the number of cancer cells that can be killed with a single radiopharmaceutical molecule.
By the mid-2010s, the Food and Drug Administration (FDA) had approved two radiopharmaceuticals that target molecules on certain B cells to treat some people with non-Hodgkin lymphoma, a type of blood cancer. But these drugs were never widely adopted. Few doctors treating patients with lymphoma were trained to administer these types of radioactive compounds. And the radiopharmaceuticals faced competition from newer, nonradioactive drugs.
The game-changer for the field came in 2018, said Jacek Capala, Ph.D., of NCI’s Radiation Research Program, when FDA approved lutetium Lu 177-dotatate (Lutathera) for the treatment of certain cancerous neuroendocrine tumors (NETs) affecting the digestive tract.
“This showed that solid tumors can also be targeted this way,” with a radiopharmaceutical built from scratch, he said. In this case, the targets are certain hormone receptors found in abundance on the surface of NET cells.
Lutetium Lu 177-dotatate was better at slowing NET growth than any previous drug tested, explained Aman Chauhan, M.D., of the University of Kentucky, who is leading several new clinical trials of the drug. “This was a huge step forward for our field,” he said.
Adapting Drugs from Imaging Compounds
Researchers are now designing and testing radiopharmaceuticals for a range of cancers as diverse as melanoma, lung cancer, colorectal cancer, and leukemia, said Dr. Capala. Any tumor that has a targetable molecule on the surface of its cells and a good blood supply—sufficient to deliver drugs—could potentially be treated with radiopharmaceuticals, added Dr. Chauhan.
Many of these newer drugs are re-engineered versions of existing compounds used for nuclear imaging. Nuclear imaging tests, such as positron emission tomography (PET), sometimes use weakly radioactive compounds linked to molecules that bind to specific targets on the surface of cancer cells. Specialized cameras can then reveal even tiny deposits of cancer cells, helping to measure the spread of cancer through the body.
Researchers have now repurposed these targeting molecules to carry more potent radioactive compounds, or isotopes, instead—ones that could kill cancer cells instead of simply helping visualize them.
Prostate cancer has been an early testing ground for this repurposing. A protein called PSMA is found in large amounts—and almost exclusively—on prostate cells. By fusing a molecule that binds to PSMA to a radioactive compound used in PET scan imaging, scientists have been able to visualize tiny deposits of prostate cancer that are too small to be detected by conventional imaging.
Several radiopharmaceutical treatments that target PSMA are now being tested in clinical trials.
Most prostate cancers are very sensitive to radiation, and external radiation is commonly used to treat the disease, explained Frank Lin, M.D., of NCI’s Center for Cancer Research, who is leading a clinical trial of one PSMA-targeting radiopharmaceutical at the NIH Clinical Center.
Most men who receive radiation as their initial treatment won’t experience a recurrence of their cancer. But if they do, it sometimes spreads throughout the body, with many small deposits of cancer cells in many organs, he explained.
“When the tumor has spread like that, you can’t really do external-beam radiation anymore, because external radiation can only be focused on and treat a small part of your body at a time,” Dr. Lin said.
Having a radiopharmaceutical that targets PSMA is a better way to give radiation in these cases, because it can be infused directly into the blood stream and circulate widely, attaching to prostate cancer cells that have spread throughout the body, he explained.
And a big advantage of having imaging and treatment molecules that use the same target is that imaging can then give doctors a sneak preview of whether the treatment is likely to work, added Dr. Lin.
For example, in Dr. Lin’s trial, men must have a PET scan with the imaging version of the compound before treatment. If the imaging compound finds its way to the cancer cells and is detected on the PET scan, then the researchers can assume that the corresponding radiopharmaceutical treatment will hit its target.
“This complementary development of diagnostics hand-in-hand with drug therapies makes this field so much more exciting,” said Dr. Chauhan. “This way we can know we’re delivering the therapy right to the tumor cells.”
Moving to Combination Therapies
While radiopharmaceuticals have shown promise in early studies, they are also, as is the case with other types of cancer drugs, unlikely to wipe out a tumor on their own.
For example, lutetium Lu 177-dotatate more than doubled the number of people who had their neuroendocrine tumors shrink after treatment, but that number was still modest: about 17%, up from 7% without the drug, explained Dr. Chauhan.
“There is still significant room for improvement,” he said.
Using radiopharmaceuticals in combination with other therapies may be one way to drive that improvement. Some researchers are now testing radiopharmaceuticals combined with radiation sensitizers—drugs that make cancer cells even more vulnerable to radiation. For example, Dr. Chauhan is leading a clinical trial of lutetium Lu 177-dotatate combined with a radiation sensitizer called triapine, which blocks cells from producing the compounds needed for DNA repair after radiation-induced damage.
In another trial, Dr. Lin is testing lutetium Lu 177-dotatate with a type of drug called a PARP inhibitor. These drugs, which are already approved to treat some types of breast, ovarian, and other cancers, block the process of DNA repair itself. “So the radiation would cause the DNA damage, and the PARP inhibitor would prevent the tumor cells from healing their DNA after the radiation,” he explained.
Other researchers are combining radiopharmaceuticals with immunotherapies to try to boost the effectiveness of these drugs. “Recent studies have shown that radiopharmaceuticals can make tumors more responsive to immunotherapy,” said Dr. Capala.
Many tumors are “cold” tumors, he explained, in that immune cells do not recognize them, or don’t work properly in the microenvironment around tumors, he explained.
But when radiation kills cancer cells, proteins and DNA from those cells can spill into the bloodstream for immune cells to see, which may allow the immune cells to recognize and kill other cancer cells throughout the body. Radiation therapy may also make the tumor microenvironment more hospitable to immune cells, added Dr. Capala.
Together, these effects can turn a cold tumor into a “hot” tumor: one that has an abundance of immune cells and may be responsive to immunotherapy drugs. Some studies have tried using external radiation to create this kind of response.
“But there’s data suggesting that [immunotherapy] works better if each tumor, each metastasis, is exposed to radiation. So radiopharmaceutical therapy has an advantage there, in that once it’s in the body it reaches all metastases,” Dr. Capala explained.
It may even make sense to combine radiopharmaceuticals with external radiation, as long as careful treatment planning can ensure a safe overall radiation dose, added Dr. Capala. “External radiation therapy is very good at targeting large tumors, and then you could combine it with radiopharmaceutical therapy to target metastases,” he said.
Challenges and Cautions
The field of radiopharmaceuticals is still in its early days. One challenge the approach will need to overcome before it can be used more widely is the shortage of doctors trained to administer such drugs.
“The number of nuclear medicine physicians in the US is small,” said Dr. Lin, who has training in both nuclear medicine and medical oncology. “And I think we only train maybe 70 or 80 new people a year.”
So far, this workforce shortage has kept radiopharmaceuticals from living up to their true potential as a personalized treatment, explained Dr. Capala. That potential reflects the fact that, unlike with other types of cancer drugs, doctors can use imaging to measure exactly how much of a radiopharmaceutical has reached a tumor, almost in real time, and adjust the dose accordingly.
But this type of treatment planning requires multidisciplinary expertise that isn’t widely available and has left people using radiopharmaceuticals more as “radioactive chemotherapy,” with a one-size-fits-all dose, he added. “This means that many patients aren’t [yet] getting optimal treatment,” said Dr. Capala.
Long-term safety studies are also needed, added Dr. Chauhan. People treated with external radiation therapy may experience some side effects, called late effects—such as the development of second cancers—months or years after treatment. Although research to date has not shown a high rate of late effects from radiopharmaceutical treatment, “these are very new agents, and we have to continue to be cautious and monitor them,” he said.
Smoothing Collaborations
Because these drugs are relatively new, even with the trials underway, “we’re just scratching the surface of drug development for radiopharmaceuticals,” Dr. Chauhan said.
In 2019, to further boost trials of promising new radiopharmaceuticals, NCI launched the Radiopharmaceutical Development Initiative (RDI) to speed promising new drugs into clinical testing.
One thing NCI hopes to achieve with the RDI is to broker more trials using combinations of drugs produced by different pharmaceutical companies that might not collaborate otherwise, explained Dr. Kunos, who is leading the initiative. Concerns about intellectual property and a lack of trust can stop such projects before they start, Dr. Kunos explained.
“These types of collaborations would not necessarily happen unless NCI was the honest broker in the middle,” he said. Right now, only about 2% of early-phase trials supported by NCI are testing radiopharmaceuticals, but with the RDI this may grow exponentially in the coming years, he added.
“We’re not going to eliminate machines or other techniques that we use in radiation therapy," Dr. Kunos said. "But with their targeted nature, we think radiopharmaceuticals are going to transform how we use radiation.”