Childhood Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Childhood Soft Tissue Sarcoma
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2020, childhood cancer mortality decreased by more than 50%.[1-3] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. For information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors, see Late Effects of Treatment for Childhood Cancer.
Pediatric soft tissue sarcomas are a heterogenous group of malignant tumors that originate from primitive mesenchymal tissue and account for 6% of all childhood tumors (rhabdomyosarcomas, 3%; other soft tissue sarcomas, 3%).[2] For more information, see the Histopathological Classification of Childhood Soft Tissue Sarcoma section.
Rhabdomyosarcoma, a tumor of striated muscle, is the most common soft tissue sarcoma in children. It accounts for 50% of the soft tissue sarcomas in children aged 0 to 14 years.[2] For more information, see Childhood Rhabdomyosarcoma Treatment.
In pediatrics, the remaining soft tissue sarcomas are commonly referred to as nonrhabdomyosarcomatous soft tissue sarcomas (NRSTS) and account for approximately 3.5% of all childhood tumors.[2,4] This summary discusses the treatment of NRSTS.
NRSTS are often classified according to the normal tissue types from which they are derived. These types include various connective tissues, peripheral nervous system tissue, smooth muscle tissue, and vascular tissue. The classification also includes undifferentiated tumors that are not clearly related to specific tissue types. For more information about vascular tumors in children, see Childhood Vascular Tumors Treatment.
Incidence of Soft Tissue Sarcoma by Age and Histology
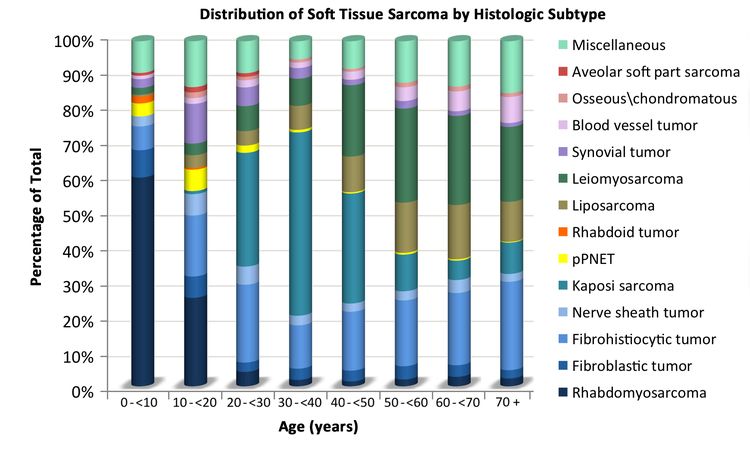
The distribution of soft tissue sarcomas by histology and age, based on the Surveillance, Epidemiology, and End Results (SEER) Program information from 2000 to 2015, is depicted in Table 1. The distribution of histological subtypes by age is also shown in Figure 2.
| Age <5 y | Age 5–9 y | Age 10–14 y | Age 15–19 y | Age <20 y | All Ages (Including Adults) | |||
|---|---|---|---|---|---|---|---|---|
| pPNET = peripheral primitive neuroectodermal tumors; SEER = Surveillance, Epidemiology, and End Results. | ||||||||
| aSource: SEER database.[5] | ||||||||
| All soft tissue and other extraosseous sarcomas | 1,124 | 773 | 1,201 | 1,558 | 4,656 | 80,269 | ||
| Rhabdomyosarcomas | 668 | 417 | 382 | 327 | 1,794 | 3,284 | ||
| Fibrosarcomas, peripheral nerve, and other fibrous neoplasms | 137 | 64 | 112 | 181 | 494 | 6,645 | ||
| Fibroblastic and myofibroblastic tumors | 114 | 33 | 41 | 77 | 265 | 4,228 | ||
| Nerve sheath tumors | 23 | 31 | 70 | 102 | 226 | 2,303 | ||
| Other fibromatous neoplasms | 0 | 0 | 1 | 2 | 3 | 114 | ||
| Kaposi sarcoma | 2 | 1 | 2 | 10 | 15 | 7,722 | ||
| Other specified soft tissue sarcomas | 237 | 238 | 559 | 865 | 1,899 | 49,004 | ||
| Ewing tumor and Askin tumor of soft tissue | 37 | 36 | 72 | 113 | 258 | 596 | ||
| pPNET of soft tissue | 24 | 23 | 42 | 56 | 145 | 402 | ||
| Extrarenal rhabdoid tumor | 75 | 8 | 9 | 4 | 96 | 205 | ||
| Liposarcomas | 4 | 6 | 37 | 79 | 126 | 10,749 | ||
| Fibrohistiocytic tumors | 43 | 73 | 142 | 223 | 481 | 13,531 | ||
| Leiomyosarcomas | 11 | 14 | 19 | 41 | 85 | 14,107 | ||
| Synovial sarcomas | 12 | 39 | 141 | 210 | 402 | 2,608 | ||
| Blood vessel tumors | 12 | 9 | 11 | 32 | 64 | 4,238 | ||
| Osseous and chondromatous neoplasms of soft tissue | 1 | 6 | 16 | 14 | 37 | 1,018 | ||
| Alveolar soft parts sarcoma | 4 | 5 | 22 | 33 | 64 | 211 | ||
| Miscellaneous soft tissue sarcomas | 14 | 19 | 48 | 60 | 141 | 1,339 | ||
| Unspecified soft tissue sarcomas | 80 | 53 | 146 | 175 | 454 | 13,614 | ||
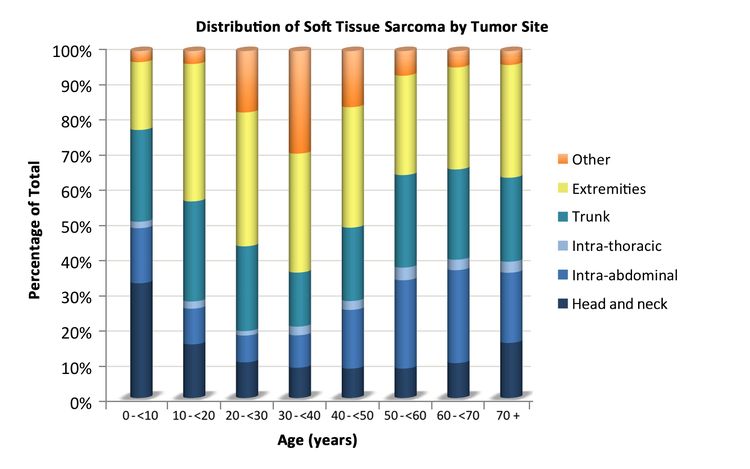
Soft tissue sarcomas include both rhabdomyosarcomas and NRSTS. NRSTS are more common in adolescents and adults.[6] Most of the information regarding treatment and natural history of the disease in younger patients has been based on studies in adult patients. The distributions of soft tissue sarcomas by age according to stage (Figure 1), histological subtype (Figure 2), and tumor site (Figure 3) are shown below.[7]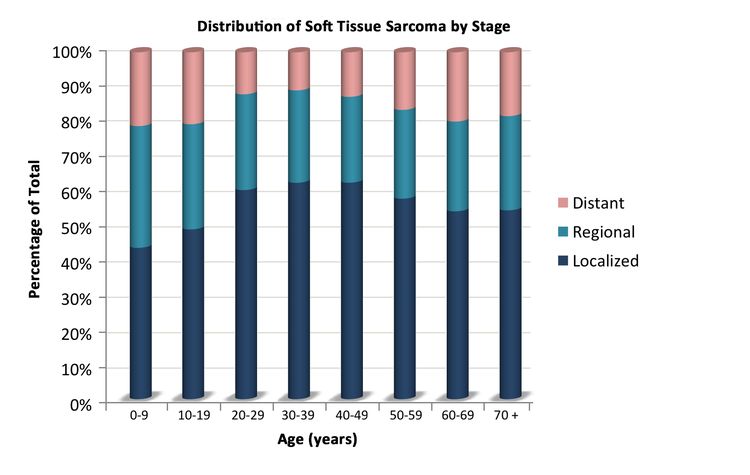
Risk Factors
Some genetic factors and external exposures have been associated with the development of NRSTS, including the following:
-
Genetic factors:
- Li-Fraumeni syndrome: Patients with Li-Fraumeni syndrome (usually resulting from heritable cancer-associated changes of the TP53 tumor suppressor gene) have an increased risk of developing soft tissue tumors (mostly NRSTS), bone sarcomas, breast cancer, brain tumors, and acute leukemia.[8,9]
- Familial adenomatous polyposis: Patients with familial adenomatous polyposis are at increased risk of developing desmoid-type fibromatosis.[10]
- RB1 gene: Germline pathogenic variants of the RB1 gene have been associated with an increased risk of developing soft tissue sarcoma, particularly leiomyosarcoma, and the risk appears higher among those younger than 1 year who were treated with alkylating agents.[11,12]
- SMARCB1 gene: Germline pathogenic variants or deletions of the SMARCB1 gene are associated with an increased risk of developing extrarenal rhabdoid tumors.[13] For more information about SMARCB1 and rhabdoid tumor predisposition syndrome type 1, see Rhabdoid Tumor Predisposition Syndrome Type 1.
- Neurofibromatosis type 1: Approximately 4% of patients with neurofibromatosis type 1 develop malignant peripheral nerve sheath tumors, which usually develop after a long latency. Some patients develop multiple lesions.[14-16]
- Werner syndrome: Werner syndrome is characterized by spontaneous chromosomal instability, resulting in increased susceptibility to cancer and premature aging. An excess of soft tissue sarcomas has been reported in patients with Werner syndrome.[17]
- Tuberous sclerosis complex: Tuberous sclerosis complex is associated with the development of various tumors showing perivascular epithelioid cell differentiation (PEComas), including lymphangioleiomyomatosis and hepatic and renal angiomyolipomas.[18-20]
- Adenosine deaminase–deficient severe combined immunodeficiency: Patients with adenosine deaminase–deficient severe combined immunodeficiency are at increased risk of developing multicentric dermatofibrosarcoma protuberans, which usually presents at an average age of 8.9 years.[21]
- External exposures:
Clinical Presentation
NRSTS can develop in any part of the body, but they arise most commonly in the trunk and extremities.[28-30] Although rare, these tumors can arise in brain tissue and are treated according to the histological type.[31]
NRSTS can present initially as an asymptomatic solid mass, or they may be symptomatic because of local invasion or impact on adjacent anatomical structures. Systemic symptoms (e.g., fever, weight loss, and night sweats) are rare. Hypoglycemia and hypophosphatemic rickets have been reported in cases of hemangiopericytoma, which was identified as a solitary fibrous tumor and is now included within myofibroma in the revised World Health Organization (WHO) classification. Hyperglycemia has been noted in patients with fibrosarcoma of the lung.[32]
Diagnostic and Staging Evaluation
When a suspicious lesion is identified, it is crucial to perform a complete workup, followed by adequate biopsy. The lesion is imaged before initiating any intervention using the following procedures:
- Plain films. Plain films can be used to rule out bone involvement and detect calcifications that may be seen in soft tissue tumors such as extraskeletal osteosarcoma or synovial sarcoma.
- Computed tomography (CT). Chest CT is essential to assess the presence of metastases. An abdominal CT can be used to image intra-abdominal tumors, such as liposarcoma. Patients with NRSTS who were treated in 11 centers as part of the European paediatric Soft Tissue Sarcoma Study Group (EpSSG) were retrospectively assessed to evaluate the impact of indeterminate pulmonary nodules identified on chest CT.[33] Of the 206 patients examined, 109 (52.9%) did not have any nodules, 78 (38%) had at least one indeterminate nodule, and 19 (9.2%) had nodules meeting the definition of metastases. The 5-year event-free survival (EFS) rate was 78.5% (95% confidence interval [CI], 69.4%–85.1%) for patients without nodules and 69.6% (95% CI, 57.9%–78.7%) for patients with indeterminate nodules (P = .135). The 5-year overall survival (OS) rate was 87.4% (95% CI, 79.3%–92.5%) for patients without nodules and 79.0% (95% CI, 67.5%–86.8%) for patients with indeterminate nodules (P = .086).
- Magnetic resonance imaging (MRI). MRI may be essential for a surgeon to achieve adequate surgical margins. MRI can be used to image intra-abdominal tumors, such as liposarcoma, and is essential for extremity lesions.
- Positron emission tomography (PET) scan and bone scan. In a retrospective study, 46 PET scans were completed in 25 pediatric patients with soft tissue sarcoma.[34] The positive predictive value of finding metastatic disease was 89%, and the negative predictive value was 67%. A small retrospective study of nine patients with NRSTS suggested that PET-CT was more accurate and cost-effective than either modality alone in identifying distant metastatic disease.[35] The use of this modality in pediatric NRSTS has not been studied prospectively.
The imaging characteristics of some tumors can be highly suggestive of that particular diagnosis. For example, the imaging characteristics of pediatric low-grade fibromyxoid sarcoma and alveolar soft part sarcoma have been described and can aid in the diagnosis of these rare neoplasms.[36]
Biopsy strategies
Although NRSTS are pathologically distinct from rhabdomyosarcoma and Ewing sarcoma, the classification of childhood NRSTS type is often difficult. Core-needle biopsy, incisional biopsy, or excisional biopsy can be used to diagnose NRSTS. If possible, the surgeon who will perform the definitive resection needs to be involved in the biopsy decision. Poorly placed incisional or needle biopsies may adversely affect the ability to achieve negative margins.
Needle biopsy techniques must ensure adequate tissue sampling. Given the diagnostic importance of translocations and other molecular changes, a core-needle biopsy or small incisional biopsy that obtains adequate tumor tissue is crucial to allow for conventional histological and immunocytochemical analysis and other studies such as light and electron microscopy, cytogenetics, fluorescence in situ hybridization, and molecular pathology.[37,38]
The acquisition of multiple cores of tissue may be required. Of 530 suspected soft tissue masses in (largely adult) patients who underwent core-needle biopsies, 426 (80%) were proven to be soft tissue tumors, 225 (52.8%) of which were malignant. Core-needle biopsy was able to differentiate soft tissue sarcomas from benign lesions with a sensitivity of 96.3% and a specificity of 99.4%. Tumor subtype was accurately assigned in 89.5% of benign lesions and in 88% of soft tissue sarcomas. The biopsy complication rate was 0.4%.[39]
Considerations related to a biopsy procedure are as follows:
- Core-needle biopsy for a deep-seated tumor can lead to formation of a hematoma, which affects subsequent resection and/or radiation (because the hematoma should be covered in the irradiated volume).
- Fine-needle biopsy is usually not recommended because it is difficult to determine the accurate histological diagnosis and grade of the tumor in this heterogeneous group of tumors.
- Image guidance using ultrasonography, CT scan, or MRI may be necessary to ensure a representative biopsy.[40] Image guidance is particularly helpful in deep lesions and to avoid cystic changes or necrotic tumors.[41]
- Incisional biopsies must not compromise subsequent wide local excision.
- Excisional biopsy of the lesion is only appropriate for small superficial lesions (<3 cm in size) and are discouraged.[42,43] If an excisional biopsy is contemplated, then MRI of the area is recommended to define the area of involvement as subsequent surgery or radiation therapy may be needed.
- Various institutional series have demonstrated the feasibility and effectiveness of sentinel lymph node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[44-49] The utility of sentinel node biopsy is currently limited to epithelioid sarcoma, clear cell sarcoma, and rhabdomyosarcoma of the trunk and extremities.[50]
In a prospective study of pediatric patients with sarcoma who underwent sentinel lymph node biopsy, 28 patients were examined. Sentinel lymph node biopsy was positive in 7 of the 28 patients, including 3 patients (43%) who had negative PET-CT scans. PET-CT overestimated and suggested nodal involvement in 14 patients, more than what was confirmed by sentinel lymph node biopsy. The findings from the sentinel lymph node biopsies resulted in altering therapy for all seven patients who were determined to have metastatic disease. As indicated by previous reports, epithelioid sarcoma and clear cell sarcoma were the two NRSTS included in this study.[50]
- In the ARST0332 (NCT00346164) study, patients with epithelioid sarcoma, clear cell sarcoma, or radiographically enlarged nodes underwent regional node sampling. Nodal metastases were identified in 20 patients (3.8%), and all but one of these patients had radiographic evidence of nodal involvement. The most common histologies included epithelioid sarcoma (18%), angiosarcoma (17%), and clear cell sarcoma (14%). Patients with isolated nodal metastases had a similar outcome to those who did not have distant metastases (5-year OS rates, 85% vs. 87%). Sentinel lymph node biopsies were encouraged but not required for this study. A sentinel lymph node biopsy was not done in most patients because they had clinically enlarged nodes. Of note, three patients without clinical evidence of lymph node metastasis at study entry experienced lymph node basin failure. One of these patients had three lymph nodes in two different lymph node basins sampled by sentinel lymph node biopsy that were pathologically negative.[51]
Transverse extremity incisions are avoided to reduce skin loss at re-excision and because they require a greater cross-sectional volume of tissue to be covered in the radiation field. Other extensive surgical procedures are also avoided before definitive diagnosis.
For these reasons, open biopsy or multiple core-needle biopsies are strongly encouraged so that adequate tumor tissue can be obtained to allow crucial studies to be performed and to avoid limiting future treatment options.
Unplanned resection
In children with unplanned resection of NRSTS, primary re-excision is frequently recommended because many patients will have tumor present in the re-excision specimen.[52,53] A single-institution analysis of adolescents and adults compared patients who had unplanned excisions of soft tissue sarcoma to stage-matched controls. In this retrospective analysis, unplanned initial excision of soft tissue sarcoma resulted in increased risk of local recurrence, metastasis, and death. This increased risk was greatest for high-grade tumors.[54][Level of evidence C1] In this case, a second resection is expected.
Chromosomal abnormalities
Many NRSTS are characterized by chromosomal abnormalities. Some of these chromosomal translocations lead to a fusion of two disparate genes. The resulting fusion transcript can be readily detected by using polymerase chain reaction–based techniques, thus facilitating the diagnosis of those neoplasms that have translocations.
Some of the most frequent aberrations seen in NRSTS are listed in Table 2.
| Histology | Chromosomal Aberrations | Genes Involved |
|---|---|---|
| aAdapted from Sandberg,[55] Slater et al.,[56] Mertens et al.,[57] Romeo,[58] and Schaefer et al.[59] | ||
| Alveolar soft part sarcoma | t(x;17)(p11.2;q25) | ASPSCR1::TFE3 [60-62] |
| Angiomatoid fibrous histiocytoma | t(12;16)(q13;p11), t(2;22)(q33;q12), t(12;22)(q13;q12) | FUS::ATF1, EWSR1::CREB1,[63] EWSR1::ATF1 |
| BCOR-rearranged sarcomas | inv(X)(p11.4;p11.2) | BCOR::CCNB3 |
| CIC-rearranged sarcomas | t(4;19)(q35;q13), t(10;19)(q26;q13) | CIC::DUX4 |
| Clear cell sarcoma | t(12;22)(q13;q12), t(2;22)(q33;q12) | EWSR1::ATF1, EWSR1::CREB1 [64] |
| Congenital (infantile) fibrosarcoma/mesoblastic nephroma | t(12;15)(p13;q25) | ETV6::NTRK3 |
| Dermatofibrosarcoma protuberans | t(17;22)(q22;q13) | COL1A1::PDGFB |
| Desmoid fibromatosis | Trisomy 8 or 20, loss of 5q21 | CTNNB1 or APC variants |
| Desmoplastic small round cell tumors | t(11;22)(p13;q12) | EWSR1::WT1 [65,66] |
| Epithelioid hemangioendothelioma | t(1;3)(p36;q25) [67] | WWTR1::CAMTA1 |
| Epithelioid sarcoma | Inactivation of SMARCB1 | SMARCB1 |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12), t(9;17)(q22;q11), t(9;15)(q22;q21), t(3;9)(q11;q22) | EWSR1::NR4A3, TAF2N::NR4A3, TCF12::NR4A3, TFG::NR4A3 |
| Hemangiopericytoma (myofibroma) | t(12;19)(q13;q13.3) and t(13;22)(q22;q13.3) | LMNA::NTRK1 [68] |
| Infantile fibrosarcoma | t(12;15)(p13;q25) | ETV6::NTRK3 |
| Inflammatory myofibroblastic tumor | t(1;2)(q23;q23), t(2;19)(q23;q13), t(2;17)(q23;q23), t(2;2)(p23;q13), t(2;11)(p23;p15) [69] | TPM3::ALK, TPM4::ALK, CLTC::ALK, RANBP2::ALK, CARS1::ALK, RAS, ROS1 [70,71] |
| Infantile myofibromatosis | Gain-of-function variants | PDGFRB [72] |
| Low-grade fibromyxoid sarcoma | t(7;16)(q33;p11), t(11;16)(p11;p11) | FUS::CREB3L2, FUS::CREB3L1 |
| Malignant peripheral nerve sheath tumor | 17q11.2, loss or rearrangement of 10p, 11q, 17q, 22q | NF1 |
| Mesenchymal chondrosarcoma | Del(8)(q13.3q21.1) | HEY1::NCOA2 |
| Myoepithelioma | t(19;22)(q13;q12), t(1;22)(q23;q12), t(6;22)(p21;q12) | EWSR1::ZNF44, EWSR1::PBX1, EWSR1::POU5F1 |
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11), t(12;22)(q13;q12) | FUS::DDIT3, EWSR1::DDIT3 |
| Primitive myxoid mesenchymal tumor of infancy | Internal tandem duplication | BCOR |
| Rhabdoid tumor | Inactivation of SMARCB1 | SMARCB1 |
| Sclerosing epithelioid fibrosarcoma | t(11;22)(p11;q12), t(19;22)(p13;q12) | EWSR1::CREB3L1, EWSR1::CREB3L3 |
| Solitary fibrous tumor | inv(12)(q13q13) | NAB2::STAT6 |
| Synovial sarcoma | t(x;18)(p11.2;q11.2) | SS18::SSX |
| Tenosynovial giant cell tumor | t(1;2)(p13;q35) | COL6A3::CSF1 |
Prognosis and Prognostic Factors
The prognosis of NRSTS varies greatly depending on the following factors:[73-75]
- Site of the primary tumor.
- Tumor size.
- Tumor grade. For more information, see the Soft Tissue Sarcoma Tumor Pathological Grading System section.
- Tumor histology.
- Depth of tumor invasion.
- Presence of metastases and site of the metastatic tumor.
- Resectability of the tumor.
- Use of radiation therapy.
In a review of a large adult series of NRSTS, patients with superficial extremity sarcomas had a better prognosis than did patients with deep tumors. This may be a reflection of differences in resectability. Thus, in addition to grade and size, the depth of invasion of the tumor should be considered.[76]
Data specific to NRSTS in children and adolescents are difficult to separate. Several adult and pediatric series have shown that patients with large or invasive tumors have a significantly worse prognosis than do those with small, noninvasive tumors. A retrospective review of soft tissue sarcomas (rhabdomyosarcoma and NRSTS) in children and adolescents suggests that the 5-cm cutoff used for adults with soft tissue sarcoma may not be ideal for smaller children, especially infants. The review identified an interaction between tumor diameter and body surface area.[77] This relationship has been questioned in a rhabdomyosarcoma study and requires further study to determine the therapeutic implications of the observation.[78]
Some pediatric NRSTS are associated with a better outcome. For instance, patients with infantile fibrosarcoma who present at age 4 years or younger have an excellent prognosis. This excellent outcome occurs because surgery alone can cure a significant number of these patients and infantile fibrosarcoma is highly chemosensitive. This tumor also responds well to larotrectinib, a specific tropomyosin receptor kinase inhibitor.[22,79]
Prognosis based on the Children's Oncology Group (COG) ARST0332 trial
Soft tissue sarcomas in older children and adolescents often behave similarly to those in adult patients.[22,80] A large, prospective, multinational COG study (ARST0332 [NCT00346164]) enrolled newly diagnosed patients younger than 30 years. Patients were assigned to treatment based on their risk group. Risk groups were defined by the presence of metastasis, tumor resectability and margins, and tumor size and grade. For more information, see Figure 4.[81][Level of evidence B4]
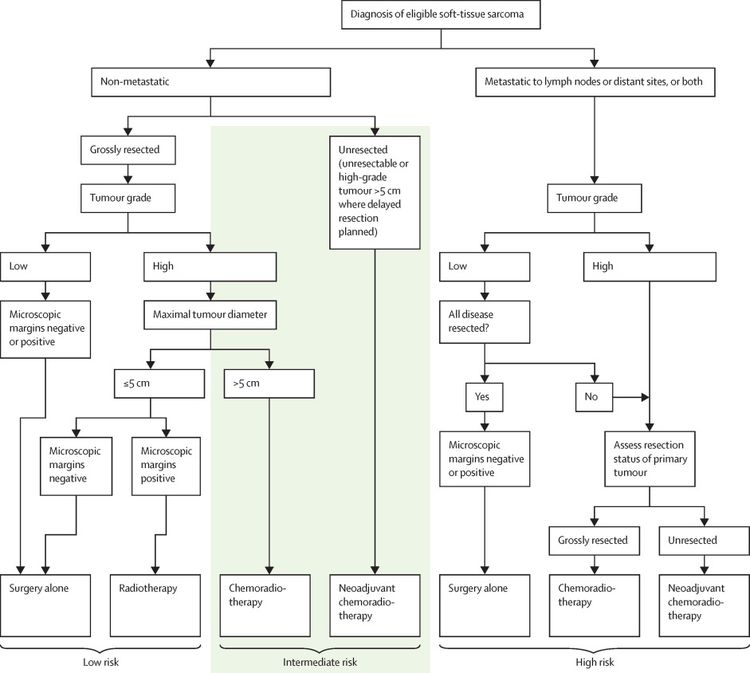
Each patient was assigned to one of three risk groups and one of four treatment groups. The risk groups were as follows:[81]
- Low risk: Nonmetastatic R0 (resection was complete with negative microscopic margins) or R1 (microscopically positive margins) low-grade tumor, or ≤5 cm R1 high-grade tumor.
- Intermediate risk: Nonmetastatic R0 or R1 >5 cm high-grade tumor, or unresected tumor of any size or grade.
- High risk: Metastatic tumor.
The treatment groups were as follows:
- Surgery alone (n = 205).
- Radiation therapy (55.8 Gy) (n = 17).
- Chemoradiation therapy (chemotherapy and 55.8 Gy radiation therapy) (n = 111).
- Neoadjuvant chemoradiation therapy (chemotherapy and 45 Gy radiation therapy, then surgery and radiation therapy boost based on margins with continued chemotherapy) (n = 196).
Chemotherapy included six cycles of ifosfamide (3 g/m2 per dose) given intravenously on days 1 through 3 and five cycles of doxorubicin (37.5 mg/m2 per dose) given intravenously on days 1 to 2 every 3 weeks, with the sequence adjusted based on the timing of surgery or radiation therapy.
For the 550 patients enrolled, 529 evaluable patients were included in the analysis. At a median follow-up of 6.5 years (interquartile range [IQR], 4.9–7.9), the survival results are shown in Table 3.
| 5-Year Event-Free Survival | 5-Year Overall Survival | |||
|---|---|---|---|---|
| Risk Group | Events/Patients | Estimate, % (95% CI) | Events/Patients | Estimate, % (95% CI) |
| CI = confidence interval; R0 = completely excised with negative microscopic margins; R1 = grossly excised but with positive microscopic margins; R2 = less than complete gross excision. | ||||
| Low | 26/222 | 88.9 (84.0–93.8) | 10/222 | 96.2 (93.2–99.2) |
| Intermediate | 84/227 | 65.0 (58.2–71.8) | 55/227 | 79.2 (73.4–85.0) |
| High | 63/80 | 21.2 (11.4–31.1) | 52/80 | 35.5 (23.6–47.4) |
| Surgical Margin | ||||
| R0 | 44/252 | 83.6 (78.3–89.0) | 22/252 | 92.8 (89.1–96.5) |
| R1 | 29/81 | 66.2 (54.8–77.5) | 17/81 | 79.7 (70.0–89.5) |
| R2 | 100/196 | 49.2 (41.4–57.0) | 78/196 | 62.7 (55.2–70.3) |
The COG ARST0332 trial was a risk-based stratification study. Overall, local control after radiation therapy was as follows: R0, 106 of 109 patients (97%); R1, 51 of 60 patients (85%); and R2/unresectable, 2 of 6 patients (33%). Local recurrence predictors included extent of delayed resection (P < .001), imaging response before delayed surgery (P < .001), histological subtype (P < .001), and no radiation therapy (P = .046). The 5-year EFS was significantly lower for patients unable to undergo R0 or R1 resection (P = .0003).[82]
Pediatric patients with unresected localized NRSTS have a poor outcome. Only about one-third of patients treated with multimodality therapy remain disease free.[73,83]; [84,85][Level of evidence C1] In an Italian review of 30 patients with NRSTS at visceral sites, only ten patients survived at 5 years. Unfavorable prognostic factors included inability to achieve complete resection, large tumor size, tumor invasion, histological subtype, and lung-pleura sites.[86][Level of evidence C1]
Prognosis based on the European paediatric Soft Tissue Sarcoma Study Group (EpSSG) NRSTS 2005 study
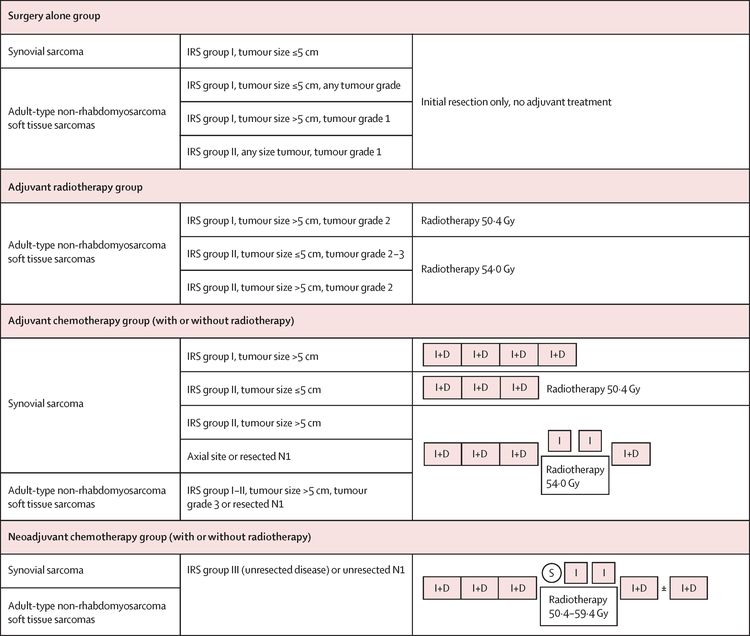
The EpSSG conducted a prospective trial for patients younger than 21 years with NRSTS. They reported an analysis of 206 patients with synovial sarcoma and 363 with adult-type NRSTS. Patients were treated according to assigned risk groups. For more information, see Figure 5.[87] With a median follow-up of 80 months (interquartile range, 54.3–111.3) for the 467 surviving patients, the 5-year EFS rate was 73.7% (95% CI, 69.7%–77.2%), and the OS rate was 83.8% (95% CI, 80.3%–86.7%). The survival by treatment groups are shown in Table 4.[87]
| Treatment Group | 5-Year Event-Free Survival Rate (95% CI) | 5-Year Overall Survival Rate (95% CI) | Local Recurrence Rate |
|---|---|---|---|
| CI = confidence interval; EpSSG = European paediatric Soft Tissue Sarcoma Study Group; NRSTS = nonrhabdomyosarcomatous soft tissue sarcomas. | |||
| Surgery alone | 91.4% (87.0%–94.4%) | 98.1% (95.0%–99.3%) | 7.6% (19/250) |
| Adjuvant radiation therapy alone (n = 17) | 75.5% (46.9%–90.1%) | 88.2% (60.6%–96.9%) | 6.7% (1/15) |
| Adjuvant chemotherapy ± radiation therapy (n = 93) | 65.6% (54.8%–74.5%) | 75.8% (65.3%–83.5%) | 10.8% (7/65) |
| Neoadjuvant chemotherapy ± radiation therapy (n = 209) | 56.4% (49.3%–63.0%) | 70.4% (63.3%–76.4%) | 14.2% (16/113) |
Treatment failures specifically for the neoadjuvant therapy treatment groups are shown in Table 5.[87]
| Treatment | Local Failure (No. of Patients) | Local + Metastatic Failure (No. of Patients) | Metastatic Failure (No. of Patients) |
|---|---|---|---|
| EpSSG = European paediatric Soft Tissue Sarcoma Study Group; No. = number; NRSTS = nonrhabdomyosarcomatous soft tissue sarcomas. | |||
| aAdapted from Ferrari et al.[87] | |||
| Radiation therapy alone (n = 21) | 7 | 2 | 4 |
| Delayed surgery followed by radiation therapy (n = 104) | 16 | 6 | 8 |
| Delayed surgery alone (n = 48) | 8 | 3 | 8 |
| No local treatment (n = 16) | 12 | 4 | 0 |
| Preoperative radiation therapy followed by delayed surgery (n = 20) | 4 | 0 | 6 |
The authors concluded that adjuvant therapy (radiation therapy and chemotherapy) could safely be omitted in the group of patients assigned to surgery alone. Their criteria included the following:[87]
- Synovial cell: Intergroup Rhabdomyosarcoma Study (IRS) group I tumor size <5 cm.
- Adult-type NRSTS: IRS group I tumor size <5 cm, any grade.
- Adult-type NRSTS: IRS group I tumor size >5 cm, tumor grade I.
- Adult-type NRSTS: IRS group II any tumor size, tumor grade I.
They also concluded that improving the outcome for patients with high-risk, initially resected, adult-type NRSTS and those with initially unresected disease remains a major clinical challenge.[87]
In a pooled analysis from U.S. and European pediatric centers, outcomes were better for patients whose tumor removal procedure was deemed complete than for patients whose tumor removal was incomplete. Outcomes were better for patients who received radiation therapy than for patients who did not.[84][Level of evidence C1]
Because long-term morbidity must be minimized while disease-free survival is maximized, the ideal therapy for each patient must be carefully and individually determined using these prognostic factors before initiating therapy.[29,88-92]
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed February 25, 2025.
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed December 30, 2024.
- Hawkins DS, Black JO, Orbach D, et al.: Nonrhabdomyosarcoma soft-tissue sarcomas. In: Blaney SM, Helman LJ, Adamson PC, eds.: Pizzo and Poplack's Pediatric Oncology. 8th ed. Wolters Kluwer, 2020, pp 721-46.
- Surveillance, Epidemiology, and End Results (SEER) Program: SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (1973-2015 varying) - Linked To County Attributes - Total U.S., 1969-2016 Counties [Database]. National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. Available online. Last accessed October 12, 2022.
- Weiss SW, Goldblum JR: General considerations. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. Mosby, 2008, pp 1-14.
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Chang F, Syrjänen S, Syrjänen K: Implications of the p53 tumor-suppressor gene in clinical oncology. J Clin Oncol 13 (4): 1009-22, 1995. [PUBMED Abstract]
- Plon SE, Malkin D: Childhood cancer and hereditary. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Lippincott Williams and Wilkins, 2015, pp 13-31.
- Groen EJ, Roos A, Muntinghe FL, et al.: Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 15 (9): 2439-50, 2008. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Wong JR, Morton LM, Tucker MA, et al.: Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol 32 (29): 3284-90, 2014. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Benign tumors of peripheral nerves. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. Mosby, 2008, pp 825-901.
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Stark AM, Buhl R, Hugo HH, et al.: Malignant peripheral nerve sheath tumours--report of 8 cases and review of the literature. Acta Neurochir (Wien) 143 (4): 357-63; discussion 363-4, 2001. [PUBMED Abstract]
- Goto M, Miller RW, Ishikawa Y, et al.: Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol Biomarkers Prev 5 (4): 239-46, 1996. [PUBMED Abstract]
- Fricke BL, Donnelly LF, Casper KA, et al.: Frequency and imaging appearance of hepatic angiomyolipomas in pediatric and adult patients with tuberous sclerosis. AJR Am J Roentgenol 182 (4): 1027-30, 2004. [PUBMED Abstract]
- Adriaensen ME, Schaefer-Prokop CM, Duyndam DA, et al.: Radiological evidence of lymphangioleiomyomatosis in female and male patients with tuberous sclerosis complex. Clin Radiol 66 (7): 625-8, 2011. [PUBMED Abstract]
- Hornick JL, Fletcher CD: PEComa: what do we know so far? Histopathology 48 (1): 75-82, 2006. [PUBMED Abstract]
- Kesserwan C, Sokolic R, Cowen EW, et al.: Multicentric dermatofibrosarcoma protuberans in patients with adenosine deaminase-deficient severe combined immune deficiency. J Allergy Clin Immunol 129 (3): 762-769.e1, 2012. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Lippincott Williams and Wilkins, 2015, pp 827-54.
- Weiss SW, Goldblum JR: Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma). In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. Mosby, 2008, pp 403-27.
- Tukenova M, Guibout C, Hawkins M, et al.: Radiation therapy and late mortality from second sarcoma, carcinoma, and hematological malignancies after a solid cancer in childhood. Int J Radiat Oncol Biol Phys 80 (2): 339-46, 2011. [PUBMED Abstract]
- Bartkowiak D, Humble N, Suhr P, et al.: Second cancer after radiotherapy, 1981-2007. Radiother Oncol 105 (1): 122-6, 2012. [PUBMED Abstract]
- Casey DL, Friedman DN, Moskowitz CS, et al.: Second cancer risk in childhood cancer survivors treated with intensity-modulated radiation therapy (IMRT). Pediatr Blood Cancer 62 (2): 311-316, 2015. [PUBMED Abstract]
- McClain KL, Leach CT, Jenson HB, et al.: Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med 332 (1): 12-8, 1995. [PUBMED Abstract]
- Dillon P, Maurer H, Jenkins J, et al.: A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg 27 (2): 241-4; discussion 244-5, 1992. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Zeytoonjian T, Mankin HJ, Gebhardt MC, et al.: Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int 25 (5): 325-30, 2004. [PUBMED Abstract]
- Benesch M, von Bueren AO, Dantonello T, et al.: Primary intracranial soft tissue sarcoma in children and adolescents: a cooperative analysis of the European CWS and HIT study groups. J Neurooncol 111 (3): 337-45, 2013. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Miscellaneous tumors of intermediate malignancy. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. Mosby, 2008, pp 1093-1160.
- Giraudo C, Schoot R, Cardoen L, et al.: Indeterminate pulmonary nodules in non-rhabdomyosarcoma soft tissue sarcoma: A study of the European paediatric Soft Tissue Sarcoma Study Group. Cancer 130 (4): 597-608, 2024. [PUBMED Abstract]
- Mody RJ, Bui C, Hutchinson RJ, et al.: FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer 54 (2): 222-7, 2010. [PUBMED Abstract]
- Tateishi U, Hosono A, Makimoto A, et al.: Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J Pediatr Hematol Oncol 29 (9): 608-12, 2007. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. Mosby, 2008.
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Strauss DC, Qureshi YA, Hayes AJ, et al.: The role of core needle biopsy in the diagnosis of suspected soft tissue tumours. J Surg Oncol 102 (5): 523-9, 2010. [PUBMED Abstract]
- Chowdhury T, Barnacle A, Haque S, et al.: Ultrasound-guided core needle biopsy for the diagnosis of rhabdomyosarcoma in childhood. Pediatr Blood Cancer 53 (3): 356-60, 2009. [PUBMED Abstract]
- Tuttle R, Kane JM: Biopsy techniques for soft tissue and bowel sarcomas. J Surg Oncol 111 (5): 504-12, 2015. [PUBMED Abstract]
- Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Williams and Wilkins, 1997.
- Smith LM, Watterson J, Scott SM: Medical and surgical management of pediatric soft tissue tumors. In: Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Williams and Wilkins, 1997, pp 360-71.
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Wagner LM, Kremer N, Gelfand MJ, et al.: Detection of lymph node metastases in pediatric and adolescent/young adult sarcoma: Sentinel lymph node biopsy versus fludeoxyglucose positron emission tomography imaging-A prospective trial. Cancer 123 (1): 155-160, 2017. [PUBMED Abstract]
- Alvarez E, He J, Spunt SL, et al.: Lymph node metastases in paediatric and young adult patients with non-rhabdomyosarcoma soft tissue sarcoma (NRSTS): Findings from Children's Oncology Group (COG) study ARST0332. Eur J Cancer 180: 89-98, 2023. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Qureshi YA, Huddy JR, Miller JD, et al.: Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol 19 (3): 871-7, 2012. [PUBMED Abstract]
- Sandberg AA: Translocations in malignant tumors. Am J Pathol 159 (6): 1979-80, 2001. [PUBMED Abstract]
- Slater O, Shipley J: Clinical relevance of molecular genetics to paediatric sarcomas. J Clin Pathol 60 (11): 1187-94, 2007. [PUBMED Abstract]
- Mertens F, Antonescu CR, Hohenberger P, et al.: Translocation-related sarcomas. Semin Oncol 36 (4): 312-23, 2009. [PUBMED Abstract]
- Romeo S, Dei Tos AP: Clinical application of molecular pathology in sarcomas. Curr Opin Oncol 23 (4): 379-84, 2011. [PUBMED Abstract]
- Schaefer IM, Cote GM, Hornick JL: Contemporary Sarcoma Diagnosis, Genetics, and Genomics. J Clin Oncol 36 (2): 101-110, 2018. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Ladanyi M: The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 4 (3): 162-73, 1995. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Antonescu CR, Dal Cin P, Nafa K, et al.: EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 46 (12): 1051-60, 2007. [PUBMED Abstract]
- Hisaoka M, Ishida T, Kuo TT, et al.: Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol 32 (3): 452-60, 2008. [PUBMED Abstract]
- Barnoud R, Sabourin JC, Pasquier D, et al.: Immunohistochemical expression of WT1 by desmoplastic small round cell tumor: a comparative study with other small round cell tumors. Am J Surg Pathol 24 (6): 830-6, 2000. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Errani C, Zhang L, Sung YS, et al.: A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50 (8): 644-53, 2011. [PUBMED Abstract]
- Haller F, Knopf J, Ackermann A, et al.: Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: a subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. J Pathol 238 (5): 700-10, 2016. [PUBMED Abstract]
- Jain S, Xu R, Prieto VG, et al.: Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol 3 (4): 416-28, 2010. [PUBMED Abstract]
- Mariño-Enríquez A, Wang WL, Roy A, et al.: Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol 35 (1): 135-44, 2011. [PUBMED Abstract]
- Lovly CM, Gupta A, Lipson D, et al.: Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4 (8): 889-95, 2014. [PUBMED Abstract]
- Agaimy A, Bieg M, Michal M, et al.: Recurrent Somatic PDGFRB Mutations in Sporadic Infantile/Solitary Adult Myofibromas But Not in Angioleiomyomas and Myopericytomas. Am J Surg Pathol 41 (2): 195-203, 2017. [PUBMED Abstract]
- Spunt SL, Hill DA, Motosue AM, et al.: Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol 20 (15): 3225-35, 2002. [PUBMED Abstract]
- Spunt SL, Poquette CA, Hurt YS, et al.: Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children's Research Hospital. J Clin Oncol 17 (12): 3697-705, 1999. [PUBMED Abstract]
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Brooks AD, Heslin MJ, Leung DH, et al.: Superficial extremity soft tissue sarcoma: an analysis of prognostic factors. Ann Surg Oncol 5 (1): 41-7, 1998 Jan-Feb. [PUBMED Abstract]
- Ferrari A, Miceli R, Meazza C, et al.: Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol 27 (3): 371-6, 2009. [PUBMED Abstract]
- Rodeberg DA, Stoner JA, Garcia-Henriquez N, et al.: Tumor volume and patient weight as predictors of outcome in children with intermediate risk rhabdomyosarcoma: a report from the Children's Oncology Group. Cancer 117 (11): 2541-50, 2011. [PUBMED Abstract]
- Hong DS, DuBois SG, Kummar S, et al.: Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21 (4): 531-540, 2020. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. Mosby, 2001.
- Spunt SL, Million L, Chi YY, et al.: A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children's Oncology Group prospective study. Lancet Oncol 21 (1): 145-161, 2020. [PUBMED Abstract]
- Million L, Hayes-Jordan A, Chi YY, et al.: Local Control For High-Grade Nonrhabdomyosarcoma Soft Tissue Sarcoma Assigned to Radiation Therapy on ARST0332: A Report From the Childrens Oncology Group. Int J Radiat Oncol Biol Phys 110 (3): 821-830, 2021. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Smith KB, Indelicato DJ, Knapik JA, et al.: Definitive radiotherapy for unresectable pediatric and young adult nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 57 (2): 247-51, 2011. [PUBMED Abstract]
- Ferrari A, Magni C, Bergamaschi L, et al.: Pediatric nonrhabdomyosarcoma soft tissue sarcomas arising at visceral sites. Pediatr Blood Cancer 64 (9): , 2017. [PUBMED Abstract]
- Ferrari A, van Noesel MM, Brennan B, et al.: Paediatric non-rhabdomyosarcoma soft tissue sarcomas: the prospective NRSTS 2005 study by the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG). Lancet Child Adolesc Health 5 (8): 546-558, 2021. [PUBMED Abstract]
- Dillon PW, Whalen TV, Azizkhan RG, et al.: Neonatal soft tissue sarcomas: the influence of pathology on treatment and survival. Children's Cancer Group Surgical Committee. J Pediatr Surg 30 (7): 1038-41, 1995. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
Histopathological Classification of Childhood Soft Tissue Sarcoma
World Health Organization (WHO) Classification of Soft Tissue Tumors
The WHO classification system for cancer represents the common nomenclature for cancer worldwide. In the United States, it has been adopted by the American Joint Committee on Cancer (AJCC) for sarcoma staging and the College of American Pathologists (CAP) cancer protocols for bone and soft tissue sarcomas. The WHO published a revision to their classification of soft tissue and bone tumors in 2020. The classification had several updates to existing classification, nomenclature, grading, and risk stratification schemes. The revised classification includes newly described entities, and it uses molecular alterations in the classifications.[1]
The grading of soft tissue tumors has always been a controversial issue. The 2020 WHO classification represents the consensus of several soft tissue pathologists and geneticists, as well as a medical oncologist, radiologist, and surgeon. This edition further integrates morphological and genetic information into the classification. For example, a new category of tumors called NTRK-rearranged spindle cell neoplasms was included, but infantile fibrosarcoma was excluded from this group. Ewing sarcoma was removed from the bone tumor section and, instead, is in the undifferentiated small cell sarcomas of bone and soft tissue section. This classification reflects the variable presentation sites and the variety of translocations seen in Ewing sarcoma. This classification also separated Ewing sarcoma from entities such as CIC-rearranged sarcomas, BCOR-rearranged sarcomas, and EWSR1 gene fusions involving non-ETS partner genes.[1]
- Adipocytic tumors.
- Benign.
- Lipoma not otherwise specified (NOS).
- Lipomatosis.
- Lipomatosis of nerve.
- Lipoblastomatosis.
- Angiolipoma NOS.
- Myolipoma.
- Chondroid lipoma.
- Spindle cell lipoma.
- Atypical spindle cell/pleomorphic lipomatous tumor.
- Hibernoma.
- Intermediate (locally aggressive).
- Malignant.
- Benign.
- Chondro-osseous tumors.
- Benign.
- Chondroma NOS.
- Malignant.
- Benign.
- Fibroblastic and myofibroblastic tumors.
- Benign.
- Nodular fasciitis.
- Proliferative fasciitis.
- Proliferative myositis.
- Myositis ossificans and fibro-osseous pseudotumor of digits.
- Ischemic fasciitis.
- Elastofibroma.
- Fibrous hamartoma of infancy.
- Fibromatosis colli.
- Juvenile hyaline fibromatosis.
- Inclusion body fibromatosis.
- Fibroma of tendon sheath.
- Desmoplastic fibroblastoma.
- Myofibroblastoma.
- Calcifying aponeurotic fibroma.
- EWSR1::SMAD3-positive fibroblastic tumor (emerging).
- Angiomyofibroblastoma.
- Cellular angiofibroma.
- Angiofibroma NOS.
- Nuchal fibroma.
- Acral fibromyxoma.
- Gardner fibroma.
- Intermediate (locally aggressive).
- Solitary fibrous tumor, benign.
- Palmar/plantar-type fibromatosis.
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Lipofibromatosis.
- Giant cell fibroblastoma.
- Intermediate (rarely metastasizing).
- Dermatofibrosarcoma protuberans NOS.
- Pigmented dermatofibrosarcoma protuberans.
- Dermatofibrosarcoma protuberans, fibrosarcomatous.
- Myxoid dermatofibrosarcoma protuberans.
- Plaque-like dermatofibrosarcoma protuberans.
- Solitary fibrous tumor NOS.
- Inflammatory myofibroblastic tumor.
- Epithelioid inflammatory myofibroblastic sarcoma.
- Myofibroblastic sarcoma.
- Superficial CD34-positive fibroblastic tumor.
- Myxoinflammatory fibroblastic sarcoma.
- Infantile fibrosarcoma.[2]
- Dermatofibrosarcoma protuberans NOS.
- Malignant.
- Solitary fibrous tumor, malignant.
- Fibrosarcoma NOS.
- Myxofibrosarcoma.
- Low-grade fibromyxoid sarcoma.[3]
- Sclerosing epithelioid fibrosarcoma.
- Benign.
- Skeletal muscle tumors.
- Benign.
- Rhabdomyoma NOS.
- Malignant.
- Rhabdomyosarcoma (embryonal, spindle cell/sclerosing, alveolar, and pleomorphic forms). For more information, see Childhood Rhabdomyosarcoma Treatment.
- Ectomesenchymoma.
- Benign.
- Smooth muscle tumors.
- Benign and intermediate.
- Leiomyoma NOS.
- Smooth muscle tumor of uncertain malignant potential.
- Malignant.
Angioleiomyoma was reclassified under perivascular tumors.
- Benign and intermediate.
- So-called fibrohistiocytic tumors.
- Benign.
- Tenosynovial giant cell tumor NOS.
- Diffuse type.
- Deep benign fibrous histiocytoma.
- Tenosynovial giant cell tumor NOS.
- Intermediate (rarely metastasizing).
- Plexiform fibrohistiocytic tumor.
- Giant cell tumor of soft parts NOS.
- Malignant.
- Malignant tenosynovial giant cell tumor.
- Benign.
- Peripheral nerve sheath tumors.
- Benign.
- Schwannoma NOS (including variants).
- Neurofibroma NOS (including variants).
- Plexiform neurofibroma.
- Perineurioma NOS.
- Granular cell tumor NOS.
- Nerve sheath myxoma.
- Solitary circumscribed neuroma.
- Meningioma NOS.
- Benign triton tumor/neuromuscular choristoma.
- Hybrid nerve sheath tumor.
- Malignant.
- Malignant peripheral nerve sheath tumor NOS.
- Malignant peripheral nerve sheath tumor, epithelioid.
- Melanotic malignant peripheral nerve sheath tumor.
- Granular cell tumor, malignant.
- Perineurioma, malignant.
- Malignant peripheral nerve sheath tumor NOS.
- Benign.
- Pericytic (perivascular) tumors.
- Benign and intermediate.
- Glomus tumor NOS (including variants).
- Glomangiomatosis.
- Myopericytoma.
- Myofibromatosis.
- Myofibroma (hemangiopericytomas are now included in recent WHO classification).
- Infantile myofibromatosis.
- Angioleiomyoma.
- Glomus tumor NOS (including variants).
- Malignant.
- Glomus tumor, malignant.
- Benign and intermediate.
- Tumors of uncertain differentiation.
- Benign.
- Myxoma NOS.
- Aggressive angiomyxoma.
- Pleomorphic hyalinizing angiectatic tumor.
- Phosphaturic mesenchymal tumor NOS.
- Perivascular epithelioid tumor, benign.
- Angiomyolipoma.
- Intermediate (locally aggressive).
- Hemosiderotic fibrolipomatous tumor.
- Angiomyolipoma, epithelioid.
- Intermediate (rarely metastasizing).
- Atypical fibroxanthoma.
- Angiomatoid fibrous histiocytoma.
- Ossifying fibromyxoid tumor NOS.
- Mixed tumor NOS.
- Mixed tumor, malignant, NOS.
- Myoepithelioma NOS.
- Malignant.
- Phosphaturic mesenchymal tumor, malignant.
- NTRK-rearranged spindle cell neoplasm (emerging).
- Synovial sarcoma NOS.
- Synovial sarcoma, spindle cell.
- Synovial sarcoma, biphasic.
- Synovial sarcoma, poorly differentiated.
- Epithelioid sarcoma.
- Alveolar soft part sarcoma.
- Clear cell sarcoma NOS.
- Extraskeletal myxoid chondrosarcoma.
- Desmoplastic small round cell tumor.
- Rhabdoid tumor NOS (extrarenal).
- Perivascular epithelioid tumor, malignant.
- Intimal sarcoma.
- Ossifying fibromyxoid tumor, malignant.
- Myoepithelial carcinoma.
- Undifferentiated sarcoma.
- Spindle cell sarcoma, undifferentiated.
- Pleomorphic sarcoma, undifferentiated.
- Intracranial mesenchymal tumor.
- Round cell sarcoma, undifferentiated.
- Benign.
- Vascular tumors.
- Benign.
- Hemangioma NOS. For more information, see Childhood Vascular Tumors Treatment.
- Intramuscular hemangioma.
- Arteriovenous hemangioma.
- Venous hemangioma.
- Epithelioid hemangioma.
- Lymphangioma NOS.
- Cystic lymphangioma.
- Acquired tufted hemangioma.
- Intermediate (locally aggressive).
- Intermediate (rarely metastasizing).
- Retiform hemangioendothelioma.
- Papillary intralymphatic angioendothelioma.
- Composite hemangioendothelioma.
- Kaposi sarcoma.
- Classic indolent Kaposi sarcoma.
- Endemic African Kaposi sarcoma.
- AIDS-associated Kaposi sarcoma.
- Iatrogenic Kaposi sarcoma.
- Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma.
- Malignant.
- Epithelioid hemangioendothelioma NOS.
- Epithelioid hemangioendothelioma with WWTR1::CAMTA1 fusion.
- Epithelioid hemangioendothelioma with YAP1::TFE3 fusion.
- Angiosarcoma.
- Epithelioid hemangioendothelioma NOS.
- Benign.
With the increased use of next-generation sequencing techniques and heightened awareness of recently approved tyrosine kinase inhibitors that target NTRK and other genes, newer subgroups of pediatric soft tissue lesions that are characterized by kinase fusions have been identified and share a similar morphological spectrum. Identifying these rare entities is important because some of them might be amenable to therapeutic targeting with novel agents. Some examples of these lesions are described below.[4]
- Lipofibromatosis-like neural tumors are superficial tumors that commonly affect children, and the cells are immunoreactive for S100. These tumors commonly have NTRK1 fusions but rarely harbor RET or ALK fusions.
- Spindle cell tumors with S100 and CD34 positivity that resemble intermediate-grade malignant peripheral nerve sheath tumors predominate in children and young adults and can affect bone and soft tissues. They have fusions in various genes, including RAF1, BRAF, NTRK1, and NTRK2.
- Infantile fibrosarcoma–like lesions morphologically resemble infantile fibrosarcoma and most commonly affect patients younger than 2 years. They have a predilection for intraabdominal sites. They often exhibit alternate fusions, involving genes such as BRAF, NTRK1, and MET.
- Spindle cell sarcomas with hemangiopericytic and myopericytic patterns can affect children and have NTRK1 fusions.
- RAF1 fusion–positive spindle cell sarcomas can be seen in children and adults and often arise in the trunk. They rarely behave aggressively.
- BRAF fusion–positive soft tissue tumors have been associated with infantile fibrosarcoma–like variants or spindle cell sarcomas that resemble malignant peripheral nerve sheath tumors. They have been reported in children and often involve the abdominal cavity.
- RET fusion–positive tumors predominantly affect children and have a similar phenotype to NTRK fusion–positive tumors. They can display fibroblastic and neural-like differentiation. These tumors are sensitive to the highly selective small-molecule RET inhibitor selpercatinib.[5]
References
- WHO Classification of Tumours Editorial Board: WHO Classification of Tumours. Volume 3: Soft Tissue and Bone Tumours. 5th ed., IARC Press, 2020.
- Steelman C, Katzenstein H, Parham D, et al.: Unusual presentation of congenital infantile fibrosarcoma in seven infants with molecular-genetic analysis. Fetal Pediatr Pathol 30 (5): 329-37, 2011. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Antonescu CR: Emerging soft tissue tumors with kinase fusions: An overview of the recent literature with an emphasis on diagnostic criteria. Genes Chromosomes Cancer 59 (8): 437-444, 2020. [PUBMED Abstract]
- Ortiz MV, Gerdemann U, Raju SG, et al.: Activity of the Highly Specific RET Inhibitor Selpercatinib (LOXO-292) in Pediatric Patients With Tumors Harboring RET Gene Alterations. JCO Precis Oncol 4: , 2020. [PUBMED Abstract]
Staging and Grading Systems for Childhood Soft Tissue Sarcoma
Assessment of disease extent has an important role in predicting the clinical outcome and determining the most effective therapy for pediatric soft tissue sarcomas. As yet, there is no well-accepted assessment system that is applicable to all childhood sarcomas. The system from the American Joint Committee on Cancer (AJCC) that is used for adults has not been validated in pediatric studies.
No standardized staging system for pediatric nonrhabdomyosarcomatous soft tissue sarcomas (NRSTS) exists, but two systems are currently used to assess disease extent:[1]
- Surgico-pathological group system: The surgico-pathological group system used by the Intergroup Rhabdomyosarcoma Study is based on the amount, or extent, of tumor that remains after initial surgery and whether the disease has metastasized. This group system was used in early pediatric trials.[2] For more information, see the Intergroup Rhabdomyosarcoma Study Clinical Group System section.
- TNM staging system: The TNM staging system is a collaborative effort between the AJCC (United States) and the International Union Against Cancer (worldwide). Staging is based on the extent of the tumor (T), the extent of spread to the lymph nodes (N), the presence of metastasis (M), and the tumor grade. For the staging of soft tissue sarcoma from the eighth edition of the AJCC Cancer Staging Manual, see Tables 6, 7, 8, and 9.[3-7] The last Children's Oncology Group (COG) trial used the sixth edition AJCC Cancer Staging Manual for soft tissue sarcoma (with central pathology review).[1] A review of children with NRSTS was performed with data from the Surveillance, Epidemiology, and End Results (SEER) Program and identified 941 patients between 1988 and 2007.[8] The COG risk stratification was validated in this cohort.
Intergroup Rhabdomyosarcoma Study Clinical Group System
Nonmetastatic disease
- Group I: Localized tumor completely resected with histologically negative margins.
- Group II: Grossly resected tumor with microscopic residual tumor at the margin(s) and/or extension into regional lymph nodes.
- Group IIA: Localized, grossly resected tumor with microscopic residual disease.
- Group IIB: Regional disease with involved nodes completely resected with no microscopic disease. The most proximal (to the patient, most distal to the tumor) regional lymph node must be negative.
- Group IIC: Regional disease with involved nodes grossly resected but with evidence of residual microscopic disease at the primary site and/or histologic involvement of the most proximal regional lymph node in the dissection.
- Group III: Localized tumor, incompletely resected, or biopsy only, with gross residual tumor.
Metastatic disease
- Group IV: Any localized or regional tumor with distant metastases present at the time of diagnosis. This includes the presence of malignant cells in effusions (pleural, peritoneal) and/or cerebrospinal fluid (rare).
Recurrent/progressive disease
- Any soft tissue sarcoma that recurs after initial treatment or progresses after radiation therapy, chemotherapy, or initial surgery.
TNM Staging System
The eighth edition of the AJCC Cancer Staging Manual has designated staging by the four criteria of tumor size, nodal status, histological grade, and metastasis and by anatomical primary tumor site (head and neck; trunk and extremities; abdomen and thoracic visceral organs; retroperitoneum; and unusual histologies and sites) (see Tables 6, 7, 8, and 9).[3-7] For information about unusual histologies and sites, see the AJCC Cancer Staging Manual.[7]
| T Category | Soft Tissue Sarcoma of the Trunk, Extremities, and Retroperitoneum | Soft Tissue Sarcoma of the Head and Neck | Soft Tissue Sarcoma of the Abdomen and Thoracic Visceral Organs |
|---|---|---|---|
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |||
| TX | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. | ||
| T1 | Tumor ≤5 cm in greatest dimension. | Tumor ≤2 cm. | Organ confined. |
| T2 | Tumor >5 cm and ≤10 cm in greatest dimension. | Tumor >2 to ≤4 cm. | Tumor extension into tissue beyond organ. |
| T2a | Invades serosa or visceral peritoneum. | ||
| T2b | Extension beyond serosa (mesentery). | ||
| T3 | Tumor >10 cm and ≤15 cm in greatest dimension. | Tumor >4 cm. | Invades another organ. |
| T4 | Tumor >15 cm in greatest dimension. | Tumor with invasion of adjoining structures. | Multifocal involvement. |
| T4a | Tumor with orbital invasion, skull base/dural invasion, invasion of central compartment viscera, involvement of facial skeleton, or invasion of pterygoid muscles. | Multifocal (2 sites). | |
| T4b | Tumor with brain parenchymal invasion, carotid artery encasement, prevertebral muscle invasion, or central nervous system involvement via perineural spread. | Multifocal (3–5 sites). | |
| T4c | Multifocal (>5 sites). | ||
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, N0 = no lymph node involvement or unknown lymph node status and N1 = lymph node involvement present. | |
| N0 | No regional lymph node metastasis or unknown lymph node status.b |
| N1 | Regional lymph node metastasis.b |
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, M0 = no metastases and M1 = metastases present. | |
| M0 | No distant metastasis.b |
| M1 | Distant metastasis.b |
| Stage | T | N | M | Grade |
|---|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||||
| aAdapted from Yoon et al. [4] and Pollock et al.[6] | ||||
| bStage IIIB for soft tissue sarcoma of the retroperitoneum; stage IV for soft tissue sarcoma of the trunk and extremities. | ||||
| IA | T1 | N0 | M0 | G1, GX |
| IB | T2, T3, T4 | N0 | M0 | G1, GX |
| II | T1 | N0 | M0 | G2, G3 |
| IIIA | T2 | N0 | M0 | G2, G3 |
| IIIB | T3, T4 | N0 | M0 | G2, G3 |
| IIIB/IVb | Any T | N1 | M0 | Any G |
| IV | Any T | Any N | M1 | Any G |
Soft Tissue Sarcoma Tumor Pathological Grading System
In most cases of soft tissue sarcomas, accurate histopathological classification alone does not yield optimal information about their clinical behavior. Therefore, several histological parameters are evaluated in the grading process, including the following:
- Degree of cellularity.
- Cellular pleomorphism.
- Mitotic activity.
- Degree of necrosis.
- Invasive growth.
This process is used to improve the correlation between histological findings and clinical outcome.[9] In children, grading of soft tissue sarcoma is complicated by certain factors, such as prognosis, patient age, extent of surgical resection, and ability to metastasize. For example, children younger than 4 years with infantile fibrosarcoma and hemangiopericytoma have a good prognosis, and angiomatoid fibrous histiocytoma and dermatofibrosarcoma protuberans can recur locally if incompletely excised but usually do not metastasize.
Testing the validity of a grading system within the pediatric population is difficult because of the rarity of these neoplasms. In 1986, the Pediatric Oncology Group (POG) conducted a prospective study on pediatric NRSTS and devised the POG grading system. Analysis of outcomes for patients with localized NRSTS demonstrated that patients with grade 3 tumors fared significantly worse than those with grade 1 or grade 2 lesions. This finding suggests that this system can accurately predict the clinical behavior of NRSTS.[9-11]
The POG and Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) grading systems have proven to be of prognostic value in pediatric and adult NRSTS.[12-16] The COG uses the FNCLCC clinically. In a study of 130 tumors from children and adolescents with NRSTS enrolled in three prospective clinical trials, a correlation was found between the POG-assigned grade and the FNCLCC-assigned grade. However, grading did not correlate in all cases; 44 patients whose tumors received discrepant grades (POG grade 3, FNCLCC grade 1 or 2) had outcomes between concurrent grade 3 and grades 1 and 2. A mitotic index of 10 or greater emerged as an important prognostic factor.[17]
The COG ARST0332 (NCT00346164) trial compared the POG and FNCLCC pathological grading systems as part of a prospective risk-based strategy. The study found that, in addition to tumor depth and invasiveness, the FNCLCC grade was strongly associated with event-free survival and overall survival.[18] The closed COG ARST1321 (NCT02180867) trial used the FNCLCC system to assign histological grade.
The FNCLCC Sarcoma Group is described below. The POG grading system is no longer used.
FNCLCC grading system
The FNCLCC histological grading system was developed for adults with soft tissue sarcoma. The purpose of the grading system is to predict which patients will develop metastasis and subsequently benefit from postoperative chemotherapy.[19,20] For information about the FNCLCC histological grading system for adults, see the FNCLCC histological grade section in Soft Tissue Sarcoma Treatment.
References
- American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. Springer, 2002.
- Maurer HM, Beltangady M, Gehan EA, et al.: The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer 61 (2): 209-20, 1988. [PUBMED Abstract]
- O'Sullivan B, Maki RG, Agulnik M, et al.: Soft tissue sarcoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 499-505.
- Yoon SS, Maki RG, Asare EA, et al.: Soft tissue sarcoma of the trunk and extremities. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 507-15.
- Raut CP, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the abdomen and thoracic visceral organs. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 517-21.
- Pollock RE, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the retroperitoneum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 531-7.
- Maki RG, Folpe AL, Guadagnolo BA, et al.: Soft tissue sarcoma - unusual histologies and sites. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 539-45.
- Waxweiler TV, Rusthoven CG, Proper MS, et al.: Non-Rhabdomyosarcoma Soft Tissue Sarcomas in Children: A Surveillance, Epidemiology, and End Results Analysis Validating COG Risk Stratifications. Int J Radiat Oncol Biol Phys 92 (2): 339-48, 2015. [PUBMED Abstract]
- Parham DM, Webber BL, Jenkins JJ, et al.: Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol 8 (7): 705-10, 1995. [PUBMED Abstract]
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Skytting B, Meis-Kindblom JM, Larsson O, et al.: Synovial sarcoma--identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand 70 (6): 543-54, 1999. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Pisters PW, Leung DH, Woodruff J, et al.: Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14 (5): 1679-89, 1996. [PUBMED Abstract]
- Coindre JM, Terrier P, Bui NB, et al.: Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 14 (3): 869-77, 1996. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Khoury JD, Coffin CM, Spunt SL, et al.: Grading of nonrhabdomyosarcoma soft tissue sarcoma in children and adolescents: a comparison of parameters used for the Fédération Nationale des Centers de Lutte Contre le Cancer and Pediatric Oncology Group Systems. Cancer 116 (9): 2266-74, 2010. [PUBMED Abstract]
- Spunt SL, Million L, Chi YY, et al.: A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children's Oncology Group prospective study. Lancet Oncol 21 (1): 145-161, 2020. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Guillou L, Coindre JM, Bonichon F, et al.: Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 15 (1): 350-62, 1997. [PUBMED Abstract]
Treatment Option Overview for Childhood Soft Tissue Sarcoma
Because of the rarity of pediatric nonrhabdomyosarcomatous soft tissue sarcomas (NRSTS), treatment should be coordinated by a multidisciplinary team that includes oncologists (pediatric or medical), pathologists, surgeons, and radiation oncologists for all children, adolescents, and young adults with these tumors. In addition, to better define the tumors' natural history and response to therapy, entry into national or institutional treatment protocols should be considered for children with rare neoplasms. Information about ongoing clinical trials is available from the NCI website.
The Children's Oncology Group (COG) performed a prospective nonrandomized trial (ARST0332 [NCT00346164]) for patients with soft tissue sarcomas.[1]
Surgical resection of the primary tumor was classified as follows:
- R0 if the resection was complete with negative microscopic margins.
- R1 if the margins were microscopically positive.
- R2 if the resection left macroscopic residual tumor.
Patients were assigned to one of the following three risk groups:
- Low risk: Nonmetastatic R0 or R1 low-grade, or ≤5 cm R1 high-grade tumor.
- Intermediate risk: Nonmetastatic R0 or R1 >5 cm high-grade, or unresected tumor of any size or grade.
- High risk: Metastatic tumor.
The treatment groups were as follows:
- Surgery alone.
- Radiation therapy (55.8 Gy).
- Chemoradiation therapy (chemotherapy and 55.8 Gy radiation therapy).
- Neoadjuvant chemoradiation therapy (chemotherapy and 45 Gy radiation therapy, then surgery and radiation therapy boost based on margins with continued chemotherapy).
Chemotherapy included six cycles of intravenous (IV) ifosfamide (3 g/m2 per dose) on days 1 through 3 and five cycles of IV doxorubicin (37.5 mg/m2 per dose) on days 1 to 2 every 3 weeks, with the sequence adjusted based on timing of surgery or radiation therapy.
The analysis included 529 evaluable patients: low risk (n = 222), intermediate risk (n = 227), and high risk (n = 80). Patients underwent surgery alone (n = 205), radiation therapy (n = 17), chemoradiation therapy (n = 111), and neoadjuvant chemoradiation therapy (n = 196).
At a median follow-up of 6.5 years (interquartile range [IQR], 4.9–7.9), the 5-year event-free survival (EFS) and overall survival (OS) rates, by risk group, were as follows:
- Low-risk group: EFS rate, 88.9% (95% confidence interval [CI], 84.0%–93.8%) and OS rate, 96.2% (95% CI, 93.2%–99.2%).
- Intermediate-risk group: EFS rate, 65.0% (95% CI, 58.2%–71.8%) and OS rate, 79.2% (95% CI, 73.4%–85.0%).
- High-risk group: EFS rate, 21.2% (95% CI, 11.4%–31.1%) and OS rate, 35.5% (95% CI, 23.6%–47.4%).
The authors concluded that pretreatment clinical features can be used to effectively define treatment failure risk and stratify young patients with NRSTS for risk-adapted therapy. Most low-risk patients can be cured without adjuvant therapy, avoiding known long-term treatment complications. Survival remains suboptimal for intermediate-risk and high-risk patients, and novel therapies are needed for these patients.
Surgery
Surgical resection of the primary tumor is the predominant therapy for most NRSTS. In the COG ARST0332 (NCT00346164) study, approximately 37% of patients younger than 30 years were treated with surgery alone.[1] Another 36% of patients had surgical resection after neoadjuvant chemoradiation therapy. Involvement of a surgeon with special expertise in the resection of soft tissue sarcomas is highly desirable.
After an appropriate biopsy and pathological diagnosis, every attempt is made to resect the primary tumor. Completeness of resection predicts outcome. In the COG ARST0332 study, complete resections with negative microscopic margins (R0) resulted in the best outcomes.[1]
- The 5-year EFS rates for patients treated with surgery and other modalities were the following:
- 84% for patients who had R0 resections.
- 66% for patients who had R1 resections.
- 49% for patients who had R2 resections.
- The 5-year OS rates for patients treated with surgery and other modalities were the following:
- 93% for patients who had R0 resections.
- 80% for patients who had R1 resections.
- 63% for patients who had R2 resections.
- The 5-year EFS rates for patients treated with surgery only were the following:
- 96% for patients with low-grade tumors who had R0 resections.
- 81% for patients with low-grade tumors who had R1 resections.
- 84% for patients with high-grade tumors that were smaller than 5 cm and had R0 resections.
The COG reported results for the subset of patients with low-grade NRSTS enrolled in the ARST0332 study.[2] Low-risk patients were treated with surgery alone. Intermediate- and high-risk patients received ifosfamide/doxorubicin and radiation therapy, with definitive resection either before or after 12 weeks of chemotherapy and radiation therapy.
| Risk Group | 5-Year Event-Free Survival Rate | 5-Year Overall Survival Rate |
|---|---|---|
| Low risk | 90% | 100% |
| Intermediate risk | 55% | 78% |
| High risk | 25% | 25% |
- In low-risk patients, local-only recurrences were seen in 10% of patients. No patients with margins of resection greater than 1 mm had local recurrences.
- Sixteen of 17 intermediate- and high-risk patients who completed neoadjuvant chemotherapy and radiation therapy underwent gross-total tumor resection, and 80% had negative margins.
- In the intermediate- and high-risk groups, events included one local recurrence and seven metastatic recurrences.
The timing of surgery depends on an assessment of the feasibility and morbidity of surgery. In the COG ARST0332 study, if the central review surgeon deemed the tumor unresectable without loss of limb, form, or function, the patient was treated in arm C with neoadjuvant radiation therapy. Surgery was performed 4 to 6 weeks after the completion of radiation therapy. This early surgery allowed for decreased morbidity, better wound healing, and more complete surgical resection. The outcomes in the COG ARST0332 study were nearly identical for intermediate-risk patients who achieved an R0 or R1 resection with up-front surgery or surgery after neoadjuvant chemoradiation therapy (70% vs. 71%, respectively). An R0 resection was more likely to occur after neoadjuvant therapy.[1] These observations are true even for high-grade tumors, where the ability to achieve R0 or R1 resections was the major predictor of EFS. Treatment with neoadjuvant chemoradiation therapy resulted in lower doses of radiation therapy and achieved greater rates of R0 resections.[3] Resectability should be determined at the time of diagnosis. While there should be an emphasis on achieving negative margins without loss of form or function, given the variability of chemosensitivity of such diverse tumors, it may be better to tailor the resection to histology for each patient.
If the initial operation fails to achieve pathologically negative tissue margins or if the initial surgery was done without the knowledge that cancer was present, a re-excision of the affected area is performed to obtain clear, but not necessarily wide, margins.[4-7] This surgical tenet is true even if no mass is detected by magnetic resonance imaging after initial surgery.[8]; [9][Level of evidence C1]
Regional lymph node metastases at diagnosis are unusual and are most often seen in patients with epithelioid and clear cell sarcomas.[10,11] Sentinel lymph node biopsy as a staging procedure in soft tissue sarcoma remains controversial. However, it may help manage selected cases in adults with clear cell sarcoma and epithelioid sarcoma. There are insufficient data to support the use of sentinel lymph node biopsy in the management of pediatric patients with other NRSTS.[12-17]
Radiation Therapy
Considerations for radiation therapy are based on the potential for surgery, with or without chemotherapy, to obtain local control without severe injury to critical organs, compromise of function, or significant cosmetic or psychological impairment. This will vary according to the following:
- Patient variables (e.g., age and sex).
- Tumor variables (e.g., histopathology, site, size, and grade).
- Surgery and subsequent margin status.
- Expectations for radiation-induced morbidities (e.g., impaired bone or muscle development, organ damage, or subsequent neoplasms).
Radiation therapy can be given preoperatively or postoperatively. It can also be used as definitive therapy in rare situations in which surgical resection is not performed.[18] Radiation field size and dose will be based on patient and tumor variables and the surgical procedure.[19] Radiation therapy is associated with improved OS compared with surgery alone when delivered preoperatively or postoperatively.[20]
Brachytherapy and intraoperative radiation may be applicable in select situations.[21-23]; [24][Level of evidence C2]
Preoperative radiation therapy
Preoperative radiation therapy has been associated with excellent local control rates.[25-27] The advantages of this approach include treating smaller tissue volumes without the need to treat a postsurgical bed and somewhat lower radiation doses because relative hypoxia from surgical disruption of vasculature and scarring is not present. Preoperative radiation therapy has been associated with an increased rate of wound complications in adults, primarily in lower extremity tumors. However, the degree of these complications is questionable.[28] Conversely, preoperative radiation therapy may lead to less fibrosis than with postoperative approaches, perhaps because of the smaller treatment volume and dose.[29] Radiation techniques, like proton-beam radiation therapy can facilitate normal tissue sparing. Compared with 3-dimensional conformal radiation therapy, intensity-modulated radiation therapy may decrease radiation dose to the skin and epiphysis when irradiating extremity sarcomas, which can translate into decreased fibrosis or growth impairment.[30,31]
Postoperative radiation therapy
Radiation therapy can also be given postoperatively. In general, radiation is indicated for patients with inadequate surgical margins and for larger, high-grade tumors.[32,33] This is particularly important in high-grade tumors with tumor margins smaller than 1 cm.[34,35]; [36][Level of evidence C3] With combined R0 (negative margin) surgery and radiation therapy, local control of the primary tumor can be achieved in about 90% of patients with extremity sarcomas, 70% to 75% of patients with retroperitoneal sarcomas, and 80% of patients overall.[21,37-40]
Retroperitoneal sarcomas are unique in that the radiosensitivity of the bowel increases the risk of injury and makes postoperative radiation therapy less desirable.[41,42] Postoperative adhesions and bowel immobility can increase the risk of damage from any given radiation dose. This contrasts with the preoperative approach in which the tumor often displaces bowel outside of the radiation field, and any exposed bowel is more mobile, which decreases exposure to specific bowel segments.
Dose and volume
Radiation volume and dose depend on the patient, tumor, and surgical variables noted above, as well as the following:
- Patient age and growth potential.
- Ability to avoid critical organs, epiphyseal plates, and lymphatics (but not the neurovascular bundles that are relatively radiation tolerant).
- Functional/cosmetic outcome.
Radiation doses are typically 45 Gy to 50 Gy preoperatively, and as high as 60 Gy to very small volumes at highest risk when postoperative resection margins are predicted to be microscopically or grossly positive. Planned brachytherapy is an option if the resection is predicted to be subtotal. This can be accomplished with a simultaneously integrated boost dose (i.e., higher dose area within the larger lower dose volume) or administered with a small field of radiation after the initial volume is treated with a dose of 45 Gy to 50 Gy. However, data documenting the efficacy of a postoperative boost to areas with microscopically positive margins are lacking.[43] The postoperative radiation dose is 55 Gy to 60 Gy for R0 resections, up to 65 Gy for R1 resections (microscopic positive margins), and higher when unresectable gross residual disease exists, depending on overall treatment goals (e.g., definitive local control vs. palliation).
The COG analyzed local recurrence (LR) for NRSTS after radiation therapy in patients treated in the ARST0332 trial.[3] Patients younger than 30 years with high-grade NRSTS received radiation therapy (55.8 Gy) for an R1, 5 cm or smaller tumor (arm B); radiation therapy (55.8 Gy) with chemotherapy for an R0/R1, larger than 5 cm tumor (arm C); or neoadjuvant radiation therapy (45 Gy) with chemotherapy plus delayed surgery, chemotherapy, and postoperative boost to 10.8 Gy for an R0, smaller than 5 mm margins tumor or R1 tumor, or 19.8 Gy for R2 or unresected tumors (arm D).
- Of 193 eligible patients, 24 had local recurrences (arm B: 1 of 15 [6.7%], arm C: 7 of 65 [10.8%], arm D: 16 of 113 [14.2%]) with a median time to local recurrence of 1.1 years (range, 0.11–5.27 years).
- Of 95 patients eligible for delayed surgery after neoadjuvant therapy, 89 (93.7%) achieved R0/R1 margins.
- Overall local control after radiation therapy were as follows: R0, 106 of 109 (97%); R1, 51 of 60 (85%); and R2/unresectable, 2 of 6 (33%).
- The authors concluded that risk-based treatment for young patients with high-grade NRSTS treated on ARST0332 produced very high local control, particularly after R0 resection (97%), despite lower-than-standard radiation therapy doses.
Radiation margins are typically 2 cm to 4 cm longitudinally and encompass fascial planes axially.[44,45]
Radiation therapy was used in the COG ARST1321 trial.
Chemotherapy
The role of postoperative chemotherapy remains unclear.[46]
Evidence (lack of clarity regarding postoperative chemotherapy):
- A meta-analysis of data from all randomized trials of adults with soft tissue sarcoma observed the following:[47]
- Recurrence-free survival was better with postoperative chemotherapy for patients with high-grade tumors larger than 5 cm.
- In a European trial, adults with completely resected soft tissue sarcoma were randomly assigned to observation or postoperative chemotherapy with ifosfamide and doxorubicin.[48][Level of evidence A1]
- Postoperative chemotherapy was not associated with improved EFS or OS.
- It is difficult to extrapolate this trial to pediatric patients because the trial included: (1) a wide variety of histologies; (2) a relatively low dose of ifosfamide; (3) patients assigned to chemotherapy had definitive radiation delayed until completion of chemotherapy; and (4) almost one-half of the patients in the trial had intermediate-grade tumors.
- In the discussion section, the authors merged their patients with previously published series, including those from the European meta-analysis, and concluded that the results suggested a benefit for postoperative chemotherapy.
- The largest prospective pediatric trial failed to demonstrate any benefit with postoperative vincristine, dactinomycin, cyclophosphamide, and doxorubicin.[37]
- Doxorubicin and ifosfamide were used in the risk-based COG ARST0332 (NCT00346164) trial.[1][Level of evidence C1]
- Although this was not a randomized study, results at 2.6 years showed that patients with high-risk (>5 cm and high grade), grossly resected, nonmetastatic tumors who were treated with radiation therapy and postoperative doxorubicin and ifosfamide had a 5-year EFS rate of 67.2% and an OS rate of 78%. However, this study did not have a comparison group of patients who did not receive chemotherapy.
- In patients with metastatic disease treated with preoperative chemotherapy and radiation therapy, the estimated 5-year EFS rate was 21.2%, and the OS rate was 35.5%.
Targeted Therapy
The use of angiogenesis and mammalian target of rapamycin (mTOR) inhibitors has been explored in the treatment of adult patients with soft tissue sarcomas but not in pediatric patients. Other targeted therapy agents are reported for specific tumor types in the following sections.
Evidence (targeted therapy in adults with soft tissue sarcoma):
- In a trial of 711 adult patients who achieved a response or stable disease after chemotherapy, patients were randomly assigned to receive ridaforolimus (a rapamycin inhibitor) or placebo.[49]
- The administration of ridaforolimus was associated with a 3-week improvement in progression-free survival (PFS) when compared with placebo.
- In another trial of 371 randomly assigned adult patients with metastatic soft tissue sarcoma that progressed after chemotherapy, pazopanib (a multitargeted receptor tyrosine kinase inhibitor) was compared with placebo.[50]
- The median PFS for the pazopanib arm was 4.6 months, compared with 1.6 months for the placebo arm. OS was not different between the two arms.
- In a study of 182 previously treated adult patients with recurrent liposarcoma, leiomyosarcoma, synovial sarcoma, and other sarcomas, patients were randomly assigned to receive either regorafenib or placebo.[51]
- Patients with nonadipocytic tumors who were treated with regorafenib had significant improvements in PFS when compared with patients who were treated with placebo.
- The COG and NRG Oncology cancer consortia conducted a randomized trial of pazopanib added to neoadjuvant chemotherapy (doxorubicin and ifosfamide) and preoperative radiation therapy in pediatric (older than 2 years) and adult patients with NRSTS. Patients with intermediate- or high-grade disease whose tumors were larger than 5 cm were eligible. The end point of the trial was pathological tumor response after adjuvant therapy. Study entry was closed early because the planned interim analysis showed that the pathological response boundary was crossed. Eighty-one patients were enrolled, but only 42 (52%) were available for response data (17 patients from each group discontinued therapy for either progression, unacceptable toxicity, or patient or physician choice).[52,53]
- Four of 18 patients (22%) in the control group had greater than 90% necrosis at resection, compared with 14 of 24 patients (58%) in the group treated with pazopanib, meeting the criteria for early stopping of the study.
- Toxicity was greater in the pazopanib group, mainly resulting from increased myelosuppression. Wound complications were also more frequent in the pazopanib group.
- With longer follow-up, the investigators were able to analyze the secondary objectives of OS and EFS.[54] At a median follow-up of 3.3 years (range, 0.1–5.8 years), the 3-year EFS rate for all patients in the intent-to-treat analysis was 52.5% for patients who received pazopanib and 50.6% for those who did not (log-rank P = .8677). The 3-year OS rate was 75.7% for patients who received pazopanib and 65.4% for the control group (log-rank P = .1919). However, the study was not powered to evaluate these end points.
References
- Spunt SL, Million L, Chi YY, et al.: A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children's Oncology Group prospective study. Lancet Oncol 21 (1): 145-161, 2020. [PUBMED Abstract]
- Douglass DP, Coffin CM, Randall RL, et al.: Clinical features and outcomes of young patients with low-grade non-rhabdomyosarcoma soft tissue sarcomas treated with a risk-based strategy: A report from Children's Oncology Group study ARST0332. Pediatr Blood Cancer 71 (8): e31062, 2024. [PUBMED Abstract]
- Million L, Hayes-Jordan A, Chi YY, et al.: Local Control For High-Grade Nonrhabdomyosarcoma Soft Tissue Sarcoma Assigned to Radiation Therapy on ARST0332: A Report From the Childrens Oncology Group. Int J Radiat Oncol Biol Phys 110 (3): 821-830, 2021. [PUBMED Abstract]
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Paulino AC, Ritchie J, Wen BC: The value of postoperative radiotherapy in childhood nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 43 (5): 587-93, 2004. [PUBMED Abstract]
- Kaste SC, Hill A, Conley L, et al.: Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop (397): 204-11, 2002. [PUBMED Abstract]
- Chandrasekar CR, Wafa H, Grimer RJ, et al.: The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 90 (2): 203-8, 2008. [PUBMED Abstract]
- Daigeler A, Kuhnen C, Moritz R, et al.: Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg 394 (2): 321-9, 2009. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Haas RL, Gronchi A, van de Sande MAJ, et al.: Perioperative Management of Extremity Soft Tissue Sarcomas. J Clin Oncol 36 (2): 118-124, 2018. [PUBMED Abstract]
- Crompton JG, Ogura K, Bernthal NM, et al.: Local Control of Soft Tissue and Bone Sarcomas. J Clin Oncol 36 (2): 111-117, 2018. [PUBMED Abstract]
- Nussbaum DP, Rushing CN, Lane WO, et al.: Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol 17 (7): 966-975, 2016. [PUBMED Abstract]
- Merchant TE, Parsh N, del Valle PL, et al.: Brachytherapy for pediatric soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 46 (2): 427-32, 2000. [PUBMED Abstract]
- Schomberg PJ, Gunderson LL, Moir CR, et al.: Intraoperative electron irradiation in the management of pediatric malignancies. Cancer 79 (11): 2251-6, 1997. [PUBMED Abstract]
- Nag S, Shasha D, Janjan N, et al.: The American Brachytherapy Society recommendations for brachytherapy of soft tissue sarcomas. Int J Radiat Oncol Biol Phys 49 (4): 1033-43, 2001. [PUBMED Abstract]
- Viani GA, Novaes PE, Jacinto AA, et al.: High-dose-rate brachytherapy for soft tissue sarcoma in children: a single institution experience. Radiat Oncol 3: 9, 2008. [PUBMED Abstract]
- Virkus WW, Mollabashy A, Reith JD, et al.: Preoperative radiotherapy in the treatment of soft tissue sarcomas. Clin Orthop (397): 177-89, 2002. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys 56 (2): 482-8, 2003. [PUBMED Abstract]
- Dickie C, Parent A, Griffin AM, et al.: The value of adaptive preoperative radiotherapy in management of soft tissue sarcoma. Radiother Oncol 122 (3): 458-463, 2017. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Rao AD, Chen Q, Million L, et al.: Preoperative Intensity Modulated Radiation Therapy Compared to Three-Dimensional Conformal Radiation Therapy for High-Grade Extremity Sarcomas in Children: Analysis of the Children's Oncology Group Study ARST0332. Int J Radiat Oncol Biol Phys 103 (1): 38-44, 2019. [PUBMED Abstract]
- Seddon B, Grange FL, Simões R, et al.: The IMRiS Trial: A Phase 2 Study of Intensity Modulated Radiation Therapy in Extremity Soft Tissue Sarcoma. Int J Radiat Oncol Biol Phys 120 (4): 978-989, 2024. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Delaney TF, Kepka L, Goldberg SI, et al.: Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys 67 (5): 1460-9, 2007. [PUBMED Abstract]
- Blakely ML, Spurbeck WW, Pappo AS, et al.: The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 34 (5): 672-5, 1999. [PUBMED Abstract]
- Skytting B: Synovial sarcoma. A Scandinavian Sarcoma Group project. Acta Orthop Scand Suppl 291: 1-28, 2000. [PUBMED Abstract]
- Hua C, Gray JM, Merchant TE, et al.: Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: the St. Jude Children's Research Hospital experience. Int J Radiat Oncol Biol Phys 70 (5): 1598-606, 2008. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Karakousis CP, Driscoll DL: Treatment and local control of primary extremity soft tissue sarcomas. J Surg Oncol 71 (3): 155-61, 1999. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 57 (3): 739-47, 2003. [PUBMED Abstract]
- Raut CP, Miceli R, Strauss DC, et al.: External validation of a multi-institutional retroperitoneal sarcoma nomogram. Cancer 122 (9): 1417-24, 2016. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Bishop AJ, Zagars GK, Torres KE, et al.: Combined Modality Management of Retroperitoneal Sarcomas: A Single-Institution Series of 121 Patients. Int J Radiat Oncol Biol Phys 93 (1): 158-65, 2015. [PUBMED Abstract]
- Al Yami A, Griffin AM, Ferguson PC, et al.: Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 77 (4): 1191-7, 2010. [PUBMED Abstract]
- Wang D, Bosch W, Kirsch DG, et al.: Variation in the gross tumor volume and clinical target volume for preoperative radiotherapy of primary large high-grade soft tissue sarcoma of the extremity among RTOG sarcoma radiation oncologists. Int J Radiat Oncol Biol Phys 81 (5): e775-80, 2011. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Ferrari A: Role of chemotherapy in pediatric nonrhabdomyosarcoma soft-tissue sarcomas. Expert Rev Anticancer Ther 8 (6): 929-38, 2008. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Woll PJ, Reichardt P, Le Cesne A, et al.: Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13 (10): 1045-54, 2012. [PUBMED Abstract]
- Demetri GD, Chawla SP, Ray-Coquard I, et al.: Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 31 (19): 2485-92, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Mir O, Brodowicz T, Italiano A, et al.: Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 17 (12): 1732-1742, 2016. [PUBMED Abstract]
- Weiss AR, Chen YL, Scharschmidt TJ, et al.: Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol 21 (8): 1110-1122, 2020. [PUBMED Abstract]
- Kayton ML, Weiss AR, Xue W, et al.: Neoadjuvant pazopanib in nonrhabdomyosarcoma soft tissue sarcomas (ARST1321): A report of major wound complications from the Children's Oncology Group and NRG Oncology. J Surg Oncol 127 (5): 871-881, 2023. [PUBMED Abstract]
- Weiss AR, Chen YL, Scharschmidt TJ, et al.: Outcomes After Preoperative Chemoradiation With or Without Pazopanib in Non-Rhabdomyosarcoma Soft Tissue Sarcoma: A Report From Children's Oncology Group and NRG Oncology. J Clin Oncol 41 (31): 4842-4848, 2023. [PUBMED Abstract]
Special Considerations for the Treatment of Children With Soft Tissue Sarcoma
Cancer in children and adolescents is rare, although the overall incidence has slowly increased since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following pediatric specialists and others to ensure that children receive treatment, supportive care, and rehabilitation to achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgeons.
- Pathologists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists and hematologists.
- Rehabilitation specialists.
- Pediatric oncology nurses.
- Social workers.
- Child-life professionals.
- Psychologists.
- Nutritionists.
For specific information about supportive care for children and adolescents with cancer, see the summaries on Supportive and Palliative Care.
The American Academy of Pediatrics has outlined guidelines for pediatric cancer centers and their role in the treatment of children and adolescents with cancer.[2] At these centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate is offered to most patients and their families. Multidisciplinary evaluation in pediatric cancer centers that have surgical and radiotherapeutic expertise is of critical importance to ensure the best clinical outcome for these patients. Although surgery with or without radiation therapy can be curative for a significant proportion of patients, the addition of chemotherapy might benefit subsets of children with the disease. Therefore, enrollment in clinical trials is encouraged. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with current standard therapy. Other types of clinical trials test novel therapies when there is no standard therapy for a cancer diagnosis. Most of the progress in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Many therapeutic strategies for children and adolescents with soft tissue tumors are similar to those for adult patients, although there are important differences. For example, the biology of the neoplasm in pediatric patients may differ dramatically from that of the adult lesion. Additionally, limb-sparing procedures are more difficult to perform in pediatric patients. The morbidity associated with radiation therapy, particularly in infants and young children, may be much greater than that observed in adults.[3]
Improved outcomes with multimodality therapy in adults and children with soft tissue sarcomas over the past 20 years have caused increasing concern about the potential long-term side effects of this therapy in children. To maximize tumor control and minimize long-term morbidity, treatment must be individualized for children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma. These patients should be enrolled in prospective studies that accurately assess any potential complications.[4]
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- American Academy of Pediatrics: Standards for pediatric cancer centers. Pediatrics 134 (2): 410-4, 2014. Also available online. Last accessed February 25, 2025.
- Suit H, Spiro I: Radiation as a therapeutic modality in sarcomas of the soft tissue. Hematol Oncol Clin North Am 9 (4): 733-46, 1995. [PUBMED Abstract]
- Hawkins DS, Black JO, Orbach D, et al.: Nonrhabdomyosarcoma soft-tissue sarcomas. In: Blaney SM, Helman LJ, Adamson PC, eds.: Pizzo and Poplack's Pediatric Oncology. 8th ed. Wolters Kluwer, 2020, pp 721-46.
Treatment of Adipocytic Tumors
Adipocytic tumors account for less than 10% of soft tissue lesions in patients younger than 20 years. The most common adipocytic tumors in children are lipomas and lipoblastomas.
Table 11 summarizes the adipocytic neoplasms seen in pediatric patients and includes information about their corresponding clinico-pathological and molecular features.[1]
| Adipocytic Tumors | Frequency [2,3] | Epidemiology | Predilection Site(s) | Histology | Cytogenetic/Molecular Alterations |
|---|---|---|---|---|---|
| M = male; F = female; HGMA2 = high-mobility group AT-hook 2; PLAG1 = pleomorphic adenoma gene 1; MDM2 = mouse double minute 2 homolog; FUS = fused in sarcoma; DDIT3 = DNA damage inducible transcript 3. | |||||
| aReprinted from Seminars in Diagnostic Pathology, Volume 36, Issue 2, Putra J, Al-Ibraheemi A, Adipocytic tumors in Children: A contemporary review, Pages 95–104, Copyright 2019, with permission from Elsevier.[1] | |||||
| Benign | |||||
| Lipoma | 64%–70% (including variants) | • Solitary: M = F | Trunk. | Monotonous sheets of mature adipocytes. | Chromosomes 12q (HMGA2), 13q and 6p. |
| • Multiple: M > F | |||||
| • Uncommon in the first 2 decades of life. | |||||
| • Most common between age 40–60 years. | |||||
| Angiolipoma | 4%–28% | • M > F | Trunk and extremities. | • Mature adipocytic proliferation. | — |
| • Most common in late teens or early twenties. | • Vascular proliferation (capillary proliferation with fibrin thrombi). | ||||
| Lipoblastoma | 18%–30% | • M > F | Trunk and extremities. | • Lobular architecture. | Chromosome 8q (PLAG1) rearrangement. |
| • Zones of maturation. | |||||
| • <3 years old (90%) | • Primitive stellate cells. | ||||
| • Multivacuolated lipoblasts. | |||||
| • Myxoid area with prominent plexiform vessels. | |||||
| Hibernoma | 2% | • M = F | Back (scapular area), chest wall, axilla and inguinal regions. | • Lobular architecture. | Chromosome 11q13-21 rearrangement. |
| • Rare in the first 2 decades of life (5%). | • Different types of cells: brown fat cells, multivacuolated lipoblasts, mature fat cells. | ||||
| • 60% occur in the 3rd and 4th decades of life. | • Prominent capillary network (less pronounced than lipoblastoma and myxoid liposarcoma). | ||||
| Intermediate | |||||
| Atypical lipomatous tumor/well-differentiated liposarcoma | Rare | • M = F | Extremities, head and neck, trunk. | • Mature adipocytic proliferation. | Supernumerary ring and giant marker chromosome 12q14-15 (MDM2). |
| • Extremely rare in children (associated with Li-Fraumeni syndrome). | • Significant variation in size. | ||||
| • Peak incidence is 6th decade of life. | • Hyperchromatic nuclei with atypia. | ||||
| Malignant | |||||
| Myxoid liposarcoma | 4% | • F > M | Extremities, trunk, head and neck and abdominal regions. | • Nodular architecture. | Recurrent t(12;16)(q13;p11) resulting in FUS::DDIT3 gene fusion. |
| • Mixture of round to spindle nonlipogenic cells and lipoblasts. | |||||
| • The most common liposarcoma in children (2nd decade of life), but less frequent than adults. | • Prominent myxoid stroma with chicken-wire vasculature. | ||||
| • Variants seen in children: pleomorphic and spindle cell subtypes. | |||||
| • Peak incidence is 4th and 5th decades of life. | • Progression to round cell morphology is uncommon in children. | ||||
| Dedifferentiated liposarcoma | Rare | • Reported in an 8-year old with a well-differentiated liposarcoma.[4] | • Lower extremity in a single case report of pediatric patient.[4] | • Transition from a well-differentiated liposarcoma to nonlipogenic, high-grade sarcoma. | Supernumerary ring and giant marker chromosome 12q14-15 (MDM2). |
| • Dedifferentiation occurs in up to 10% of well-differentiated liposarcomas in adults. | • Retroperitoneum (adults). | • Heterologous differentiation (5%–10%). | |||
| • Peak incidence is 6th decade of life. | |||||
| Pleomorphic liposarcoma | Rare/not reported | • Peak incidence of pleomorphic liposarcoma is 7th decade of life. | • Extremities (adults). | • Pleomorphic lipoblasts. | — |
| • The subtype has been reported in the settings of Li-Fraumeni [5] and Muir-Torre syndromes.[6] | • Background of a high-grade, pleomorphic sarcoma (non-lipogenic). | ||||
Liposarcoma, Well-Differentiated, Not Otherwise Specified (NOS)
Liposarcoma is rare in the pediatric population and accounts for 3% of soft tissue sarcoma in patients younger than 20 years (see Table 1).
In a review of 182 pediatric patients with adult-type sarcomas, only 14 had a diagnosis of liposarcoma.[7] One retrospective study identified 34 patients younger than 22 years from 1960 to 2011.[8] There were roughly equal numbers of male and female patients, and the median age was 18 years. In an international clinico-pathological review, the characteristics of 82 cases of pediatric liposarcoma were reported.[9] The median age was 15.5 years, and females were more commonly affected. In both reports, most patients had myxoid liposarcoma.[8,9]
A literature review of 275 cases of pediatric liposarcoma showed that:[10]
- Myxoid liposarcoma was the most common histology (68%), followed by well-differentiated liposarcoma (10.5%).
- Twelve percent of patients died of disease, and most of the deaths occurred in patients with the pleomorphic and myxoid pleomorphic subtypes.
- About 70% of patients with myxoid and well-differentiated liposarcoma were treated with surgery only. The overall clinical outcomes for these groups of patients were excellent, with no evidence of disease in 114 of 127 patients.
- In contrast, more than 50% of patients with pleomorphic liposarcoma received radiation therapy and chemotherapy in addition to surgery, and their clinical outcome was suboptimal, with no evidence of disease in only 5 of 10 patients.
- Germline TP53 pathogenic variants were seen in two patients with myxoid pleomorphic liposarcoma and two patients with well-differentiated liposarcoma who had a family history compatible with Li-Fraumeni syndrome.
Clinical presentation
Most liposarcomas in the pediatric and adolescent age range are low grade and located subcutaneously. Metastasis to lymph nodes is uncommon, and most metastases are pulmonary. Tumors arising in the periphery are more likely to be low grade and myxoid. Tumors arising centrally are more likely to be high grade, pleomorphic, and present with metastasis or recur with metastasis.
Histopathological classification
The World Health Organization (WHO) classification for liposarcoma is as follows:[11]
- Intermediate (locally aggressive).
- Atypical lipomatous tumor. These tumors do not metastasize unless they undergo dedifferentiation.
- Malignant.
- Dedifferentiated liposarcoma.
- Myxoid liposarcoma. Pure myxoid liposarcomas are characterized by a t(12;16)(q13;p11) translocation and can metastasize, but patients usually have an excellent outcome when they do not have a round cell component.[12] Myxoid liposarcoma is the most common subtype of liposarcoma in the pediatric population.[8,9]
- Pleomorphic liposarcoma. This is an uncommon type of liposarcoma and primarily arises in older adults.
- Myxoid pleomorphic liposarcoma. This rare entity occurs primarily in children, adolescents, and young adults. It commonly presents in the mediastinum and is clinically aggressive.
- Liposarcoma, well-differentiated, NOS.
Genomic alterations
- Atypical lipomatous tumor. This entity is characterized by supernumerary ring and giant marker chromosomes that contain chromosomal region 12q14-q15, which includes MDM2. MDM2 amplification can be detected in virtually all cases of atypical lipomatous tumor/well-differentiated liposarcoma, with nearby genes such as CDK4 and FRS2 commonly being coamplified with MDM2.[13]
- Dedifferentiated liposarcoma. This entity, like atypical lipomatous tumor, is characterized by MDM2 amplification and the supernumerary ring and giant marker chromosomes containing the chromosomal region 12q14-q15. Dedifferentiated liposarcoma contains a high number of segmental copy number alterations, but has few gene variants.[14]
- Myxoid liposarcoma. This entity is characterized by the t(12;16)(q13;p11) translocation that produces the FUS::DDIT3 gene fusion.[14] In a small percentage of cases, EWSR1 substitutes for FUS, producing the EWSR1::DDIT3 gene fusion (t(12;22)(q13;q12)). DDIT3 (previously called CHOP and GADD153) is a stress-induced gene that has an inhibitory effect on adipogenesis.[15] Myxoid liposarcoma is the most common subtype of liposarcoma in the pediatric population. Most pediatric cases show the FUS::DDIT3 gene fusion.[8,9,16]
- Pleomorphic liposarcoma. This entity is primarily a disease of older adults and lacks either DDIT3 gene rearrangements or MDM2 amplification. Cases of pleomorphic liposarcoma typically have multiple chromosomal imbalances, including variants in TP53 and NF1 observed in some cases.[17]
- Myxoid pleomorphic liposarcoma. This entity most commonly presents in the adolescent and young adult population and lacks the DDIT3 gene rearrangement of myxoid liposarcoma and the MDM2 amplification of atypical lipomatous tumor and dedifferentiated liposarcoma.[9,16,18] Instead, myxoid pleomorphic liposarcoma presents with multiple chromosomal gains and losses. Loss of Rb expression is commonly observed, sometimes in association with loss of chromosome 13q14 where RB1 is located.[18,19] Although most cases of myxoid pleomorphic liposarcoma lack TP53 variants, a minority have TP53 variants that are associated with Li-Fraumeni syndrome in some cases.[20-22]
Prognosis
Higher grade or central tumors are associated with a significantly higher risk of death. In an international retrospective review, the 5-year survival rate was 42% for patients with central tumors. Seven of ten patients with pleomorphic myxoid liposarcoma died of their disease.[9] In a retrospective study of 14 patients, the 5-year survival rate was 78%. Tumor grade, histological subtype, and primary location correlated with survival.[8]
Treatment of liposarcoma
Treatment options for liposarcoma include the following:
- Surgery. If the tumor is not completely removed or locally recurs, a second surgery may be performed.[23-25]
- Chemotherapy followed by surgery.
- Surgery and radiation therapy (evidence based on adult studies).[26,27]
- Targeted therapy (evidence based on adult studies).[28]
Surgery
Surgery is the most important treatment for liposarcoma. After complete surgical resection of well-differentiated or myxoid liposarcoma, the event-free survival (EFS) and overall survival (OS) rates are roughly 90%.[29] If initial surgery is incomplete, re-excision should be performed to achieve a wide margin of resection.[23-25] Local recurrences have been seen and are controlled with a second resection of the tumor, particularly for low-grade liposarcomas.
Chemotherapy
Chemotherapy has been used to decrease the size of liposarcoma before surgery to facilitate complete resection, particularly in central tumors.[30,31] The role of postoperative chemotherapy for liposarcoma is poorly defined. Postoperative therapy for completely resected myxoid liposarcomas does not appear to be needed. Even with the use of postoperative chemotherapy, the survival of patients with pleomorphic liposarcomas remains poor.[32]
There are limited data to support the use of trabectedin in pediatric patients.[33] Trabectedin has produced encouraging responses in adults with advanced myxoid liposarcoma.[34] In one study, adult patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[35][Level of evidence B1]
Treatment with eribulin, a nontaxane microtubule dynamics inhibitor, significantly improved survival in adult patients with recurrent liposarcoma compared with dacarbazine. The median OS was 15.6 months for patients who received eribulin, versus 8.4 months for patients who received dacarbazine. Survival differences were more pronounced in patients with dedifferentiated and pleomorphic liposarcoma. Eribulin was effective in prolonging survival of patients with either high-grade or intermediate-grade tumors.[36][Level of evidence A1] A pediatric phase I trial of eribulin did not accrue any patients with liposarcoma.[37]
Surgery and radiation therapy
Radiation therapy is also considered either preoperatively or postoperatively, depending on the cosmetic/functional consequences of additional surgery and radiation therapy.[38,39]
Targeted therapy
In a phase II, single-arm, multicenter study, 41 adult patients with unresectable or metastatic high-grade or intermediate-grade liposarcoma were treated with pazopanib at a dose of 800 mg daily.[28][Level of evidence B4]
- The progression-free survival (PFS) rate at 12 weeks was 68.3%, which was significantly greater than the null hypothesis value of 40%.
- Forty-four percent of patients experienced tumor control. One patient had a partial response, and 17 patients had stable disease.
- At 24 weeks, 39% of the patients remained progression free. The median PFS was 4.4 months, and median OS was 12.6 months.
References
- Putra J, Al-Ibraheemi A: Adipocytic tumors in Children: A contemporary review. Semin Diagn Pathol 36 (2): 95-104, 2019. [PUBMED Abstract]
- Coffin CM, Alaggio R: Adipose and myxoid tumors of childhood and adolescence. Pediatr Dev Pathol 15 (1 Suppl): 239-54, 2012. [PUBMED Abstract]
- Dehner LP, Gru AA: Nonepithelial Tumors and Tumor-like Lesions of the Skin and Subcutis in Children. Pediatr Dev Pathol 21 (2): 150-207, 2018 Mar-Apr. [PUBMED Abstract]
- Yozu M, Symmans P, Dray M, et al.: Muir-Torre syndrome-associated pleomorphic liposarcoma arising in a previous radiation field. Virchows Arch 462 (3): 355-60, 2013. [PUBMED Abstract]
- Palit A, Inamadar AC: Circumferential skin folds in a child: a case of Michelin tire baby syndrome. Indian J Dermatol Venereol Leprol 73 (1): 49-51, 2007 Jan-Feb. [PUBMED Abstract]
- Goucha S, Khaled A, Zéglaoui F, et al.: Nevus lipomatosus cutaneous superficialis: Report of eight cases. Dermatol Ther (Heidelb) 1 (2): 25-30, 2011. [PUBMED Abstract]
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Sidebotham EL, et al.: Prognostic factors and survival in pediatric and adolescent liposarcoma. Sarcoma 2012: 870910, 2012. [PUBMED Abstract]
- Alaggio R, Coffin CM, Weiss SW, et al.: Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 33 (5): 645-58, 2009. [PUBMED Abstract]
- Baday YI, Navai SA, Hicks MJ, et al.: Pediatric liposarcoma: A case series and literature review. Pediatr Blood Cancer 68 (12): e29327, 2021. [PUBMED Abstract]
- Fletcher CDM, Bridge JA, Hogendoorn P, et al., eds.: WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press, 2013.
- Sreekantaiah C, Karakousis CP, Leong SP, et al.: Cytogenetic findings in liposarcoma correlate with histopathologic subtypes. Cancer 69 (10): 2484-95, 1992. [PUBMED Abstract]
- Kanojia D, Nagata Y, Garg M, et al.: Genomic landscape of liposarcoma. Oncotarget 6 (40): 42429-44, 2015. [PUBMED Abstract]
- Powers MP, Wang WL, Hernandez VS, et al.: Detection of myxoid liposarcoma-associated FUS-DDIT3 rearrangement variants including a newly identified breakpoint using an optimized RT-PCR assay. Mod Pathol 23 (10): 1307-15, 2010. [PUBMED Abstract]
- Han J, Murthy R, Wood B, et al.: ER stress signalling through eIF2α and CHOP, but not IRE1α, attenuates adipogenesis in mice. Diabetologia 56 (4): 911-24, 2013. [PUBMED Abstract]
- Peng R, Li N, Lan T, et al.: Liposarcoma in children and young adults: a clinicopathologic and molecular study of 23 cases in one of the largest institutions of China. Virchows Arch 479 (3): 537-549, 2021. [PUBMED Abstract]
- Barretina J, Taylor BS, Banerji S, et al.: Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet 42 (8): 715-21, 2010. [PUBMED Abstract]
- Creytens D, Folpe AL, Koelsche C, et al.: Myxoid pleomorphic liposarcoma-a clinicopathologic, immunohistochemical, molecular genetic and epigenetic study of 12 cases, suggesting a possible relationship with conventional pleomorphic liposarcoma. Mod Pathol 34 (11): 2043-2049, 2021. [PUBMED Abstract]
- Hofvander J, Jo VY, Ghanei I, et al.: Comprehensive genetic analysis of a paediatric pleomorphic myxoid liposarcoma reveals near-haploidization and loss of the RB1 gene. Histopathology 69 (1): 141-147, 2016. [PUBMED Abstract]
- Sinclair TJ, Thorson CM, Alvarez E, et al.: Pleomorphic myxoid liposarcoma in an adolescent with Li-Fraumeni syndrome. Pediatr Surg Int 33 (5): 631-635, 2017. [PUBMED Abstract]
- Francom CR, Leoniak SM, Lovell MA, et al.: Head and neck pleomorphic myxoid liposarcoma in a child with Li-Fraumeni syndrome. Int J Pediatr Otorhinolaryngol 123: 191-194, 2019. [PUBMED Abstract]
- Zare SY, Leivo M, Fadare O: Recurrent Pleomorphic Myxoid Liposarcoma in a Patient With Li-Fraumeni Syndrome. Int J Surg Pathol 28 (2): 225-228, 2020. [PUBMED Abstract]
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Samuels BL, Chawla SP, Somaiah N, et al.: Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer 123 (23): 4640-4647, 2017. [PUBMED Abstract]
- La Quaglia MP, Spiro SA, Ghavimi F, et al.: Liposarcoma in patients younger than or equal to 22 years of age. Cancer 72 (10): 3114-9, 1993. [PUBMED Abstract]
- Ferrari A, Casanova M, Spreafico F, et al.: Childhood liposarcoma: a single-institutional twenty-year experience. Pediatr Hematol Oncol 16 (5): 415-21, 1999 Sep-Oct. [PUBMED Abstract]
- Cecchetto G, Alaggio R, Dall'Igna P, et al.: Localized unresectable non-rhabdo soft tissue sarcomas of the extremities in pediatric age: results from the Italian studies. Cancer 104 (9): 2006-12, 2005. [PUBMED Abstract]
- Huh WW, Yuen C, Munsell M, et al.: Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer 57 (7): 1142-6, 2011. [PUBMED Abstract]
- Baruchel S, Pappo A, Krailo M, et al.: A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the Children's Oncology Group. Eur J Cancer 48 (4): 579-85, 2012. [PUBMED Abstract]
- Gronchi A, Bui BN, Bonvalot S, et al.: Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 23 (3): 771-6, 2012. [PUBMED Abstract]
- Demetri GD, von Mehren M, Jones RL, et al.: Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 34 (8): 786-93, 2016. [PUBMED Abstract]
- Demetri GD, Schöffski P, Grignani G, et al.: Activity of Eribulin in Patients With Advanced Liposarcoma Demonstrated in a Subgroup Analysis From a Randomized Phase III Study of Eribulin Versus Dacarbazine. J Clin Oncol 35 (30): 3433-3439, 2017. [PUBMED Abstract]
- Schafer ES, Rau RE, Berg S, et al.: A phase 1 study of eribulin mesylate (E7389), a novel microtubule-targeting chemotherapeutic agent, in children with refractory or recurrent solid tumors: A Children's Oncology Group Phase 1 Consortium study (ADVL1314). Pediatr Blood Cancer 65 (8): e27066, 2018. [PUBMED Abstract]
- Lee ATJ, Thway K, Huang PH, et al.: Clinical and Molecular Spectrum of Liposarcoma. J Clin Oncol 36 (2): 151-159, 2018. [PUBMED Abstract]
- Beane JD, Yang JC, White D, et al.: Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 21 (8): 2484-9, 2014. [PUBMED Abstract]
Treatment of Chondro-osseous Tumors
Chondro-osseous tumors have several subtypes, including the following:
Extraskeletal Mesenchymal Chondrosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcomas in patients younger than 20 years (see Table 1). Mesenchymal chondrosarcoma is more common in the head and neck region.
Histopathological features and genomic alterations
Mesenchymal chondrosarcoma is a rare tumor characterized by small round cells and hyaline cartilage, and it more commonly affects young adults.
Mesenchymal chondrosarcoma has been associated with a consistent chromosomal rearrangement. A retrospective analysis of cases of mesenchymal chondrosarcoma identified a HEY1::NCOA2 gene fusion in 10 of 15 tested specimens.[1] This gene fusion was not associated with chromosomal changes that could be detected by karyotyping. In one instance, translocation t(1;5)(q42;q32) was identified in a case of mesenchymal chondrosarcoma and shown to be associated with a novel IRF2BP::CDX1 gene fusion.[2]
A retrospective study analyzed 13 patients with mesenchymal chondrosarcoma, all with confirmed HEY1::NCOA2 gene fusions.[3]
- The median age of presentation was 19 years.
- Five patients with mesenchymal chondrosarcomas (39%) had an intraosseous presentation (skull, maxilla, palate, and mandible), while the remaining eight cases occurred in the brain/meninges, orbit, and nasal cavity.
- Microscopically, head and neck mesenchymal chondrosarcomas were characterized by primitive round cells arranged in a distinctive nested architecture and a rich staghorn vasculature.
- A cartilaginous component of hyaline cartilage islands and/or single chondrocytes were present in 69% of cases.
- A combined immunoprofile of CD99(+)/SATB2(+)/CD34(-)/STAT6(-) was typically noted.
Prognostic factors and prognosis
A retrospective survey of European institutions identified 113 children and adults with mesenchymal chondrosarcoma. Factors associated with better outcomes included the following:[4][Level of evidence C1]
- Lack of metastatic disease at initial presentation.
- Clear resection margins.
- Administration of postoperative chemotherapy after resection for patients with initially localized disease.
A retrospective analysis of Surveillance, Epidemiology, and End Results (SEER) Program data from 1973 to 2011 identified 205 patients with mesenchymal chondrosarcoma; 82 patients had skeletal primary tumors, and 123 patients had extraskeletal tumors.[5] The outcomes of patients with skeletal and extraskeletal primary tumors were the same. Factors associated with outcomes included the following:
- Primary site: The 5-year overall survival (OS) rate was 50% for patients with appendicular tumors, 37% for patients with axial tumors, and 74% for patients with cranial tumors.
- Metastases and tumor size: Presence of metastatic disease and larger tumor size were independently associated with an increased risk of death.
A single-institution retrospective review identified 43 cases of mesenchymal chondrosarcoma from 1979 to 2010.[6] Thirty patients with localized disease were evaluated. The mean age at diagnosis was 33 years (range, 11–65 years).
- The 5-year OS rate was 51%, and the 10-year OS rate was 37%.
- Younger age (<30 years) and male sex were associated with poorer OS and disease-free survival (DFS).
- Patients who did not receive adjuvant radiation therapy were more likely to have a local recurrence.
Treatment of extraskeletal mesenchymal chondrosarcoma
Treatment options for extraskeletal mesenchymal chondrosarcoma include the following:
A review of 15 patients younger than 26 years included 11 patients with soft tissue lesions from the German Cooperative Soft Tissue Sarcoma Study Group and 4 patients with primary bone lesions from the German-Austrian-Swiss Cooperative Osteosarcoma Study Group protocols. The review suggested that complete surgical removal, or incomplete resection followed by radiation therapy, was necessary for local control.[9][Level of evidence C1]
A single-institution, retrospective review identified 12 pediatric patients with mesenchymal chondrosarcoma.[10] Eleven patients presented with localized disease, and one patient presented with pulmonary nodules. Six patients received preoperative chemotherapy. All patients received postoperative chemotherapy (most commonly ifosfamide/doxorubicin) with or without radiation therapy (median dose, 59.4 Gy).
- The NCOA2 rearrangement was documented in these patients' tumors.
- The study confirmed that surgical resection is necessary for cure.
- At a median follow-up of 4.8 years, the 5-year DFS rate was 68.2% (95% confidence interval [CI], 39.8%–96.6%), and the OS rate was 88.9% (95% CI, 66.9%–100%).
A Japanese study of patients with extraskeletal myxoid chondrosarcoma and mesenchymal chondrosarcoma randomly assigned patients to treatment with either trabectedin or best supportive care.[11] The median age of patients was 38 years (range, 21–77 years).
- The OS was higher for the patients assigned to receive trabectedin than for patients assigned to receive best supportive care.
Osteosarcoma, Extraskeletal
Extraskeletal osteosarcoma is extremely rare in the pediatric and adolescent population. Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcomas in patients younger than 20 years (see Table 1).
Genomic alterations
A review of 32 adult patients with extraskeletal osteosarcomas consistently revealed several alterations.[12] Frequent genomic alterations included copy number losses in CDKN2A (70%), TP53 (56%), and RB1 (49%). Variants were identified that affected methylation/demethylation (40%), chromatin remodeling (27%), and the WNT/SHH pathways (27%). Cases with simultaneous TP53 and RB1 biallelic copy number losses were associated with worse DFS and OS.
Prognostic factors and prognosis
Extraskeletal osteosarcoma is associated with a high risk of local recurrence and pulmonary metastasis.[13]
A single-institution retrospective review identified 43 patients with extraskeletal osteosarcoma; 37 patients had localized disease, and 6 patients presented with metastatic disease. The median age was 55 years (range, 7–81 years). Seventy-five percent of patients received chemotherapy.[14]
- The median progression-free survival (PFS) was 21 months.
- The median OS was 50 months.
- There was a trend toward better survival for patients who received chemotherapy, and a statistically significant improvement in survival for patients who received chemotherapy that included cisplatin.
In a review of 274 patients with extraskeletal osteosarcoma, the median age at diagnosis was 57 years (range, 12–91 years).[15][Level of evidence C1]
- The 5-year DFS and OS rates were significantly better for those who received chemotherapy.
- The use of an osteosarcoma-type regimen was associated with improved response rates.
The European Musculoskeletal Oncology Society performed a retrospective analysis of 266 eligible patients with extraskeletal osteosarcoma treated between 1981 and 2014. Fifty patients (19%) presented with metastatic disease.[15]
- An analysis of the 211 patients who achieved complete remission after surgical resection of the primary tumor showed a 5-year OS rate of 51% and a DFS rate of 43%.
- There was a favorable trend for survival among patients who were treated with chemotherapy that is usually employed for patients with osseous osteosarcoma.
- In a multivariable analysis, factors associated with better prognosis included younger age (<40 years), smaller tumors, and use of chemotherapy.
An analysis of SEER Program data from 1973 to 2009 identified 256 patients (6%) with extraskeletal osteosarcoma among 4,173 patients with high-grade osteosarcoma.[16]
- Compared with skeletal osteosarcoma, patients with extraskeletal osteosarcoma were more likely to be older, female, have an axial primary tumor, and have regional lymph node involvement.
- Adverse prognostic features included presence of metastatic disease, larger tumor size, older age, and axial primary tumor site.
Treatment of extraskeletal osteosarcoma
Treatment options for extraskeletal osteosarcoma include the following:
Typical chemotherapy regimens used for osteosarcoma include some combination of cisplatin, doxorubicin, high-dose methotrexate, and ifosfamide.[13-15]
For more information about the treatment of extraosseous osteosarcoma, including chemotherapy options, see Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment.
References
- Wang L, Motoi T, Khanin R, et al.: Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 51 (2): 127-39, 2012. [PUBMED Abstract]
- Nyquist KB, Panagopoulos I, Thorsen J, et al.: Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One 7 (11): e49705, 2012. [PUBMED Abstract]
- Xu B, Rooper LM, Dermawan JK, et al.: Mesenchymal chondrosarcoma of the head and neck with HEY1::NCOA2 fusion: A clinicopathologic and molecular study of 13 cases with emphasis on diagnostic pitfalls. Genes Chromosomes Cancer 61 (11): 670-677, 2022. [PUBMED Abstract]
- Frezza AM, Cesari M, Baumhoer D, et al.: Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 51 (3): 374-81, 2015. [PUBMED Abstract]
- Schneiderman BA, Kliethermes SA, Nystrom LM: Survival in Mesenchymal Chondrosarcoma Varies Based on Age and Tumor Location: A Survival Analysis of the SEER Database. Clin Orthop Relat Res 475 (3): 799-805, 2017. [PUBMED Abstract]
- Kawaguchi S, Weiss I, Lin PP, et al.: Radiation therapy is associated with fewer recurrences in mesenchymal chondrosarcoma. Clin Orthop Relat Res 472 (3): 856-64, 2014. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Bishop MW, Somerville JM, Bahrami A, et al.: Mesenchymal Chondrosarcoma in Children and Young Adults: A Single Institution Retrospective Review. Sarcoma 2015: 608279, 2015. [PUBMED Abstract]
- Morioka H, Takahashi S, Araki N, et al.: Results of sub-analysis of a phase 2 study on trabectedin treatment for extraskeletal myxoid chondrosarcoma and mesenchymal chondrosarcoma. BMC Cancer 16: 479, 2016. [PUBMED Abstract]
- Jour G, Wang L, Middha S, et al.: The molecular landscape of extraskeletal osteosarcoma: A clinicopathological and molecular biomarker study. J Pathol Clin Res 2 (1): 9-20, 2016. [PUBMED Abstract]
- Sordillo PP, Hajdu SI, Magill GB, et al.: Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer 51 (4): 727-34, 1983. [PUBMED Abstract]
- Paludo J, Fritchie K, Haddox CL, et al.: Extraskeletal Osteosarcoma: Outcomes and the Role of Chemotherapy. Am J Clin Oncol 41 (9): 832-837, 2018. [PUBMED Abstract]
- Longhi A, Bielack SS, Grimer R, et al.: Extraskeletal osteosarcoma: A European Musculoskeletal Oncology Society study on 266 patients. Eur J Cancer 74: 9-16, 2017. [PUBMED Abstract]
- Thampi S, Matthay KK, Boscardin WJ, et al.: Clinical Features and Outcomes Differ between Skeletal and Extraskeletal Osteosarcoma. Sarcoma 2014: 902620, 2014. [PUBMED Abstract]
Treatment of Fibroblastic and Myofibroblastic Tumors
Fibroblastic and myofibroblastic tumors have several subtypes, including the following:
- Intermediate (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Intermediate (rarely metastasizing).
- Dermatofibrosarcoma protuberans not otherwise specified (NOS).
- Inflammatory myofibroblastic tumor.
- Epithelioid inflammatory myofibroblastic sarcoma.
- Infantile fibrosarcoma.
- Malignant.
Desmoid-Type Fibromatosis
Desmoid-type fibromatosis has previously been called desmoid tumor or aggressive fibromatosis.
Desmoid-type fibromatosis has an extremely low potential to metastasize. The tumors are locally infiltrating, and surgical control can be challenging because of difficulty obtaining margins of resection that contain the entire infiltrating tumor.
Desmoid-type fibromatosis has a high potential for local recurrence. These tumors also have a highly variable natural history, including well documented examples of spontaneous regression.[1,2]
Genomic alterations
Most desmoid tumors are sporadic, but a small proportion may occur in association with a variant in the APC gene (associated with intestinal polyps and a high incidence of colon cancer). In a study of 519 patients older than 10 years with a diagnosis of desmoid-type fibromatosis, 39 patients (7.5%, a possible underestimation) were found to have familial adenomatous polyposis (FAP).[3] The patients with FAP and desmoid-type fibromatosis were younger, more often male, and had more abdominal wall or mesenteric tumors than did patients with desmoid-type fibromatosis without FAP.
Variants in exon 3 of the CTNNB1 gene are seen in more than 80% of desmoid-type fibromatosis cases. The 45F variant in exon 3 of the CTNNB1 gene has been associated with an increased risk of disease recurrence.[4]
Currently, there are no general recommendations for genetic testing in children with desmoid-type fibromatosis. Pathological and molecular characteristics of the tumor only provide guidance for screening.
A patient should be referred to a genetic counselor if there is a family history of colon cancer, congenital hyperplasia of the retinal pigment epithelium is present,[5,6] or the desmoid-type fibromatosis occurs in the abdomen or abdominal wall.[3] If the tumor has a somatic CTNNB1 variant, screening is not necessary, because the APC gene variant has not been described in this setting. If a CTNNB1 variant is not identified, screening for the APC variant may be warranted.[7,8]
Pediatric desmoid tumors can harbor additional variants in the AKT, BRAF V600E, TP53, and RET genes.[9] For more information, see the Familial Adenomatous Polyposis (FAP) section in Genetics of Colorectal Cancer.
Treatment of desmoid-type fibromatosis
Evaluating the benefit of treatment interventions for desmoid-type fibromatosis has been extremely difficult, because desmoid-type fibromatosis has a highly variable natural history, with partial regressions seen in up to 20% of patients.[2] Large adult series and smaller pediatric series have reported long periods of disease stabilization and even regression without systemic therapy.[10,11]; [12][Level of evidence C2] For instance, in a large placebo-controlled trial of sorafenib in adult patients with desmoid tumors, the patients who received no therapy (observation/placebo) demonstrated a 20% partial regression rate, and 46% of the patients in the placebo group had no progression at 1 year.[2]
Treatment options for desmoid-type fibromatosis include the following:
Observation
Because of the variable natural history of desmoid tumors, as outlined above, observation is sometimes a viable option. This is particularly the case for lesions that are asymptomatic, do not pose a danger to vital organs, or are incompletely resected.[11,13-19]
A global consensus meeting that involved sarcoma experts with experience in both adult and pediatric desmoid tumor was organized to define the appropriate management of these tumors. The Desmoid Tumor Working Group suggested that an initial active surveillance approach does not influence the efficacy of subsequent treatments. They suggested that active therapy should only be considered in cases of persistent progression or symptoms. Active surveillance includes continuous monitoring with a first magnetic resonance imaging within 1 to 2 months of diagnosis, followed by scans in 3- to 6-month intervals. When the disease is located in critical structures that may pose significant morbidity, such as the mesentery and head and neck region, earlier treatment decisions should be made.[20]
Evidence (observation vs. initial surgery):
- A nonrandomized prospective cohort study included 771 patients with desmoid-type fibromatosis who were referred for second opinion and molecular analysis from 22 referral centers in France. The study compared initial surgery with initial observation.[21][Level of evidence C2]
- There was no difference in event-free survival (EFS) rates between the two groups (53% vs. 58%; P = .415).
- Among patients with favorable tumor locations (defined as abdominal wall, intra-abdominal, breast, digestive viscera, and lower limb), the 2-year EFS rate was similar in patients who underwent surgery (70%) or were observed (63%; P = .41).
- Among patients with tumors in unfavorable locations (defined as chest wall, head and neck, and upper limb), the 2-year EFS rate was significantly better for those treated nonsurgically (52%) compared with those who underwent initial surgery (25%; P = .001).
- There were 173 patients with desmoid-type fibromatosis who were treated on European paediatric Soft Tissue Sarcoma Study Group (EpSSG) studies since 2005. Thirteen patients (8%) had biopsies only (no further treatment), 65 patients (42%) received chemotherapy only, 31 patients (20%) underwent surgery only, 36 patients (23%) had both chemotherapy and surgery, and 9 patients (6%) received radiation therapy in addition to other therapies.[22][Level of evidence C2]
- All patients were alive at the time of analysis.
- The authors concluded that the conservative nonsurgical approach did not compromise outcome in pediatric patients.
Chemotherapy, for unresectable or recurrent tumors
The following chemotherapy regimens have been used to treat desmoid-type fibromatosis:
- Methotrexate and vinblastine: This combination produced objective responses in about one-third of patients with unresectable or recurrent desmoid-type fibromatosis.[23]
- Doxorubicin and dacarbazine: A series of mainly adult patients with FAP and unresectable desmoid-type fibromatosis that were unresponsive to hormone therapy showed that doxorubicin plus dacarbazine followed by meloxicam (an NSAID) can be safely administered and can induce responses.[24]
- Pegylated liposomal doxorubicin: In a study of 11 patients, 4 patients achieved an objective response and 7 patients had stable disease.[25] In a series of five patients, a median progression-free interval of 29 months was reported.[26]
- Hydroxyurea: A retrospective analysis reported the results of 16 children with previously treated desmoid tumors who were treated with hydroxyurea. Before hydroxyurea, seven patients had tumor progression, two patients had increased pain, and seven patients had both. Tumor shrinkage occurred in 37.5% of patients (with 18.7% partial remissions), and symptom improvement occurred in 68.7% of patients.[27]
- Vinorelbine: A retrospective review of 24 patients with desmoid-type fibromatosis (median age, 13.9 years; range, 1–23 years) received oral vinorelbine at eight centers of the Société Française des Cancers de l'Enfant between 2005 and 2020. For the 23 evaluable patients, 13% had partial responses, 78% had disease stabilization, and 9% had disease progression. The progression-free survival (PFS) rate was 89.3% at 24 months.[28]
Tyrosine kinase inhibitors
Targeted therapy has been used to treat children and adults with desmoid-type fibromatosis.
Evidence (sorafenib):
- An international, prospective, phase III, double-blind study was conducted through the National Clinical Trials Network to evaluate the efficacy of sorafenib in patients with unresectable progressive or symptomatic desmoid tumors. Eighty-seven patients were enrolled (aged 18–72 years). Patients were randomly assigned in a 2:1 fashion to receive either sorafenib or placebo. Crossover to sorafenib was permitted after disease progression.[2][Level of evidence B1]
- The objective response rate was 33% (95% confidence interval [CI], 20%–48%) in the sorafenib arm and 20% (95% CI, 8%–38%) in the placebo arm.
- The median time to objective response was 9.5 months for patients treated with sorafenib and 13.3 months for patients who received the placebo.
- The 2-year PFS rate was 81% for patients treated with sorafenib, compared with 36% for patients who received the placebo.
Evidence (pazopanib):
- One small series included six patients (aged 3–21 years) with desmoid-type fibromatosis who were treated with pazopanib.[29]
- Symptomatic improvement and stable disease were reported in all patients.
- A randomized noncomparative study included adult patients with desmoid tumors who were treated with either pazopanib or methotrexate/vinblastine.[30]
- About 84% of the patients who received pazopanib had no progression at 6 months.
NOTCH pathway/gamma-secretase inhibitors
The NOTCH pathway has been implicated in the development of desmoid tumors.[31] The NOTCH pathway/gamma-secretase inhibitor nirogacestat has been evaluated in adult and pediatric patients.
Evidence (nirogacestat):
- One study included 17 adult patients with desmoid tumors, 15 of whom had variants in the APC or CTNNB1 genes.[32][Level of evidence C3]
- Five patients (29%) achieved a confirmed partial response to nirogacestat.
- Four adult patients experienced grade 1 irregular menstruation.
- In a clinical trial (NCT03785964), 75% of women of childbearing potential reported events related to ovarian dysfunction.[33]
- A small series included four patients younger than 20 years who received nirogacestat on a compassionate basis.[34][Level of evidence C3]
- Three patients had a durable benefit, defined as a complete response (n = 1), partial response (n = 1), or stable disease (n = 1).
- No patients experienced grades 3 or 4 adverse events.
- The U.S. Food and Drug Administration (FDA) approved nirogacestat for the treatment of patients aged 18 years and older with progressive desmoid tumors who require systemic therapy. The approval was based on a prospective, randomized, placebo-controlled trial conducted by a consortium.[33]
- In 142 patients, nirogacestat had a significant PFS benefit over placebo (hazard ratio for disease progression or death, 0.29; 95% CI, 0.15–0.55; P < .001).
- The likelihood of being event free at 2 years was 76% with nirogacestat and 44% with placebo.
- Nirogacestat was associated with significant benefits related to PFS, objective response, pain, symptom burden, physical functioning, role functioning, and health-related quality of life in adults with progressing desmoid tumors.
- Nirogacestat was associated with a significant risk of ovarian failure.
NSAIDs
NSAIDs such as sulindac have been used in single cases for desmoid-type fibromatosis. The responses seen were usually disease stabilization.[35]
Antiestrogen treatment
Antiestrogen treatment, usually tamoxifen, plus sulindac has also resulted in disease stabilization.[36] A prospective trial of the combination of tamoxifen and sulindac reported few side effects, although asymptomatic ovarian cysts were common in girls. This combination showed relatively little activity, as measured by rates of response and PFS.[37][Level of evidence B4]
Surgery
Surgical resection should be used judiciously in patients with desmoid tumors because spontaneous regression can occur in up to 20% of cases. Surgical resection is recommended when tumor enlargement threatens the airway or when symptoms such as pain are persistent. A watch-and-wait strategy is otherwise preferred.
If surgery is chosen, the intent is to achieve clear margins. However, a retrospective review of children who underwent surgery for desmoid-type fibromatosis at St. Jude Children’s Research Hospital (SJCRH) reported no correlation between surgical margins and risk of recurrence.[19] In this study, 10 of 39 patients experienced a recurrence after surgery, with a median interval time of 2.5 years.
Radiation therapy
Radiation has been used for unresectable and symptomatic desmoid-type fibromatosis or postoperatively for tumors with inadequate resections if progression would have morbid consequences. The potential long-term complications of radiation therapy, especially subsequent neoplasms, make this modality less appealing in younger patients.[38]
Postoperative radiation therapy can be considered when recurrence or progression would entail additional surgery that might cause functional or cosmetic compromise and if radiation is considered acceptable in terms of morbidities.
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Dermatofibrosarcoma Protuberans NOS
Dermatofibrosarcoma is a rare tumor that can be present in all age groups, but many of the reported cases arise in children.[39-41] A review of 451 cases in children younger than 20 years in the SEER Program database found that the incidence was 1 case per 1 million. The incidence was highest among Black patients aged 15 to 19 years. The most common sites were the trunk and extremities, which is similar to what is found in adults.
Ninety-five percent of patients underwent surgery. The overall survival (OS) rate was 100% at 5 years, 98% at 15 years, and 97% at 30 years. Male patients had decreased survival compared with female patients (P < .05).[42][Level of evidence C1]
Genomic alterations
The tumor has a consistent chromosomal translocation t(17;22)(q22;q13) that juxtaposes the COL1A1 gene with the PDGFRB gene.
Treatment of dermatofibrosarcoma protuberans
Guidelines for workup and management of dermatofibrosarcoma protuberans have been published.[43]
Treatment options for dermatofibrosarcoma protuberans include the following:
Surgery with or without radiation therapy
Most patients with dermatofibrosarcoma tumors can be cured by complete surgical resection. Wide excision with negative margins or Mohs/modified-Mohs surgery will prevent most tumors from recurring.[44] Despite the locally aggressive behavior of the tumor, lymph node or visceral metastasis rarely occurs.
Evidence (surgery):
- The EpSSG prospective NRSTS 2005 (NCT00334854) trial identified 46 patients with dermatofibrosarcoma protuberans.[45] The median age at diagnosis was 6.9 years (range, 0.4–17.5 years). All patients had localized disease, 93% of patients had small tumors (<5 cm), and 76% of patients had Intergroup Rhabdomyosarcoma Study (IRS) group I tumors.
- All patients underwent up-front surgery, and 32 patients required two procedures.
- There were 11 patients with IRS group II tumors, 2 of whom went on to have a local recurrence.
- After a median follow-up of 49 months (range, 4.2–130.9 months), all patients were alive at the time of this report.
- The 5-year EFS rate was 92.6% (95% CI, 78.8%–97.6%), and the OS rate was 100%.
In retrospective reviews, postoperative radiation therapy after incomplete excision may have decreased the likelihood of recurrence.[46,47] Metastatic disease is more likely after multiple recurrences, and radiation or other adjuvant therapy should be considered in patients with recurrences that cannot be managed surgically.[40,42]
Targeted therapy (imatinib)
When surgical resection cannot be accomplished or the tumor is recurrent, treatment with imatinib (a tyrosine kinase inhibitor) has been effective in adult patients.[48-50]
Evidence (imatinib):
- A systematic review of nine studies examined 152 adult patients with histologically proven dermatofibrosarcoma protuberans who were treated with imatinib.[51]
- The study demonstrated a complete response rate of 5.2%, a partial response rate of 55.2%, and a stable disease rate of 27.6%.
- There were no differences in the response rates based on imatinib dosing of either 400 mg or 800 mg per day.
Inflammatory Myofibroblastic Tumor and Epithelioid Inflammatory Myofibroblastic Sarcoma
Inflammatory myofibroblastic tumor is a rare mesenchymal tumor that is more common in children and adolescents.[52-54]
Clinical presentation
Inflammatory myofibroblastic tumors are rare tumors that affect soft tissues and visceral organs of children and young adults.[55] They rarely metastasize but tend to be locally invasive. Usual anatomical sites of disease include soft tissue, lungs, spleen, colon, and breast.[52] A review of 42 cases of pediatric inflammatory myofibroblastic tumor of the bladder was published in 2015.[56]
Epithelioid inflammatory myofibroblastic sarcoma is an uncommon subtype of inflammatory myofibroblastic tumors that shows a male predominance and can present from infancy through adulthood.[57-59] This subtype shows epithelioid morphology and a perinuclear or nuclear membrane pattern of immunostaining for ALK.[57] The most common site of presentation is the abdomen, although other primary sites have been reported.[57-59]
Genomic alterations
Roughly one-half of inflammatory myofibroblastic tumors exhibit a clonal variant that activates the ALK gene (encodes a receptor tyrosine kinase) at chromosome 2p23.[60]
Most cases of epithelioid inflammatory myofibroblastic sarcoma have RANBP2::ALK gene fusions. RRBP1::ALK gene fusions have also been reported.[57-59] Because RANBP2 localizes to the nuclear pore, this likely explains the perinuclear or nuclear membrane pattern of staining noted for ALK in epithelioid inflammatory myofibroblastic sarcoma.
ROS1 and PDGFRB kinase fusions were identified in 8 of 11 patients (73%) who were negative for ALK by immunohistochemistry.[61][Level of evidence C3]
Prognosis
Inflammatory myofibroblastic tumors recur frequently but are rarely metastatic.[52-54] Studies of children with inflammatory myofibroblastic tumor show 5-year survival rates higher than 80%.[62]
Epithelioid inflammatory myofibroblastic sarcoma is an aggressive tumor that is generally treated with surgery. Before the availability of ALK inhibitors, disease progression and high mortality rates were common.[57,58,63] Epithelioid inflammatory myofibroblastic sarcoma generally responds to ALK inhibitors but progression on therapy has been observed, which is consistent with the aggressive clinical behavior of the tumor.[59]
Treatment of inflammatory myofibroblastic tumor
Treatment options for inflammatory myofibroblastic tumor include the following:
Surgery and chemotherapy
Complete surgical removal, when feasible, is the mainstay of therapy.[64]
Evidence (surgery with or without chemotherapy):
- In a series of nine patients, the following was observed:[65][Level of evidence C1]
- Four patients achieved continuous remission after complete resection.
- Three patients with residual disease experienced disease recurrence but later achieved continuous remission.
- One patient with metastatic disease responded to multiagent chemotherapy.
- In another study of 31 patients who underwent complete surgical resection, 4 patients had local disease recurrences.[62]
- Of the 4 patients with local recurrences, all patients were alive after surgical re-resection (3 patients) or adjuvant chemotherapy and resection (1 patient).
- A review of German studies identified 37 patients younger than 21 years with inflammatory myofibroblastic tumors.[66][Level of evidence C1]
- Of 20 patients, 17 had complete resections with no recurrences. Surgical resections can be limited to those procedures that preserve form and function.
- All other patients were treated with a combination of surgery and various chemotherapy regimens.
- The overall 5-year EFS rate was 75%, and the OS rate was 91%.
- A series of 32 patients aged 18 years and younger found that complete excision was the mainstay of therapy, although some patients were treated with steroids or cytotoxic chemotherapy.[67][Level of evidence C1]
- The OS rate was 94%.
- Three patients had relapsed disease, two of whom died of the disease.
- When complete excision was performed, with or without other treatments such as steroids, there was a high survival rate for these patients.
The benefit of chemotherapy has been noted in case reports.[68] A prospective registry of children with inflammatory myofibroblastic tumor from the EpSSG (2005–2016) found an EFS rate of 82.9% and an OS rate of 98.1% at 5 years in all patients. The response rate for patients who received systemic therapy (chemotherapy or ALK inhibitor therapy) was 63% when used as front-line therapy and 66% when used as second-line therapy. Eight of ten patients who received vinblastine and low-dose methotrexate and all five patients who received ALK inhibitors (all of whom had ALK-positive tumors) responded to treatment.[62]
A retrospective, international, multicenter study analyzed patients younger than 21 years with ROS1-altered inflammatory myofibroblastic tumors who were enrolled in either the EpSSG NRSTS-2005 study or the Soft Tissue Sarcoma Registry. Primary surgery was recommended if a microscopic radical resection without disfigurement was feasible. Of the 19 patients, 12 received neoadjuvant systemic therapy as first-line treatment (high-dose steroids, n = 2; vinorelbine/vinblastine with methotrexate, n = 6; ROS1 inhibitors, n = 8). With a median follow-up of 2.8 years, seven patients had an event. The 3-year EFS rate was 41% (95% CI, 11%–71%), and the OS rate was 100%. While many patients in this series received crizotinib, the specific ROS1 inhibitor used for each patient was not specified.[69]
Steroid therapy or NSAID therapy
There are case reports of response to either steroids or NSAIDs.[62,70,71]
Targeted therapy (ALK inhibitors)
Inflammatory myofibroblastic tumors respond to ALK inhibitor therapy, as follows:
Crizotinib
Evidence (crizotinib):
- For pediatric patients with measurable disease, the use of crizotinib achieved partial tumor responses in three of six patients with ALK-translocated inflammatory myofibroblastic tumors.[72]
- A case report of a patient aged 16 years with metastatic/multifocal ALK-positive inflammatory myofibroblastic tumor demonstrated a complete response and a 3-year disease-free interval with crizotinib therapy.[73]
- One study included 14 patients with inflammatory myofibroblastic tumors who were treated with crizotinib.[74][Level of evidence C3]
- Five patients had complete responses, seven had partial responses, and the remaining two had stable disease.
- No patient had relapsed disease at the time the article was published.
- Two adult patients with ALK-rearranged inflammatory myofibroblastic tumor achieved partial responses with crizotinib.[75][Level of evidence C3]
- An extensive review confirmed the effectiveness of crizotinib in children with inflammatory myofibroblastic tumors in various locations.[76]
The FDA approved crizotinib for patients aged 1 year and older with unresectable, recurrent, or refractory inflammatory ALK-positive myofibroblastic tumors.
Ceritinib
Evidence (ceritinib):
- Two pediatric patients enrolled in a clinical trial responded to treatment with ceritinib.[77]
- One patient had a complete response that was durable for multiple years on continuing therapy.
- The other patient had a partial response when the drug was discontinued for severe liver and renal toxicity.
- In a multicenter phase I study of ceritinib, 7 of 10 patients with inflammatory myofibroblastic tumor had objective responses to ceritinib.[78]
- In a phase I trial of ceritinib for adult patients who were previously treated with ALK inhibitors, one patient with inflammatory myofibroblastic tumor had a partial response.[79]
Alectinib
A case report described the successful treatment of a patient with an inflammatory myofibroblastic tumor and a FN1::ALK gene fusion using alectinib, a second-generation ALK inhibitor.[80]
For information about the treatment of this tumor in the lungs, see Childhood Pulmonary Inflammatory Myofibroblastic Tumors Treatment.
Infantile Fibrosarcoma
There are two distinct types of fibrosarcoma in children and adolescents, as follows:
- Infantile fibrosarcoma is a malignant fibroblastic tumor usually characterized by ETV6::NTRK3 gene fusions.
- Adult-type fibrosarcoma is composed of monomorphic fibroblastic tumor cells. Some of the genomic features of adult-type fibrosarcoma have been recently described.[81]
These are two distinct pathological diagnoses and require different treatments.
Clinical presentation
Infantile fibrosarcoma usually occurs in children younger than 1 year. This tumor usually presents with a rapidly growing mass, often noted at birth or even seen in the prenatal ultrasound. The tumors are frequently quite large at the time of presentation.[82] Hypercalcemia secondary to elevated levels of parathyroid hormone–related protein has been reported as a presenting feature of this disease in newborns.[83]
These tumors have a low incidence of metastases at diagnosis.
Genomic alterations
The tumor usually has a characteristic cytogenetic translocation t(12;15)(p13;q25) to create the ETV6::NTRK3 fusion gene. Infantile fibrosarcoma shares this translocation and a virtually identical histological appearance with mesoblastic nephroma.
Infantile fibrosarcoma occasionally occurs in children up to age 4 years. A tumor with similar morphology has been identified in older children. In these older children, the tumors do not have the ETV6::NTRK3 fusion that is characteristic of the tumors in younger patients.[84] BRAF intragenic deletions have been described in cases of infantile fibrosarcoma. Some of these tumors also contained NTRK3 fusions.[85] One study described four young children (aged 2–10 years) with tumors that were histologically classified as infantile fibrosarcoma and had ALK rearrangements.[86]
The Associazione Italiana Ematologia Oncologia Pediatrica analyzed a cohort of 44 pediatric patients with tumors classified as infantile fibrosarcomas/congenital mesoblastic nephromas. Eight infantile fibrosarcoma–like mesenchymal tumors found to be negative for the ETV6::NTRK3 fusion gene were analyzed by RNA sequencing to identify novel driver events. They identified three fusion genes involving RAF1: GOLGA4::RAF1, LRRFIP2::RAF1, and CLIP1::RAF1. The three fusion proteins retained the entire catalytic domain of the RAF1 kinase.[87]
Treatment of infantile fibrosarcoma
Treatment options for infantile fibrosarcoma include the following:
Surgery, observation, and/or chemotherapy
Complete resection is curative in most patients with infantile fibrosarcoma. However, the large size of the lesion frequently makes resection without major functional consequences impossible. For instance, tumors of the extremities often require amputation for complete excision.
The European pediatric group has reported that observation may also be an option in patients with microscopic residual disease after surgery.[88] Twelve patients with microscopic residual disease received no further therapy and two patients experienced disease relapse. One patient obtained a complete remission after chemotherapy. Postoperative chemotherapy was administered to patients with higher group disease and those who progressed. In a subsequent study, only one of seven patients with microscopic residual disease progressed during observation. That patient achieved complete remission with chemotherapy.[89][Level of evidence C1]
Preoperative chemotherapy has made a more conservative surgical approach possible. Agents active in this setting include vincristine, dactinomycin, cyclophosphamide, and ifosfamide.[90,91]; [89,92,93][Level of evidence C1] Three older studies of patients with infantile fibrosarcoma suggested that an alkylator-free regimen was effective and used as the first treatment choice in patients with macroscopic disease.[88,89,94] However, newer results of studies using NTRK inhibitors have suggested that kinase inhibitors are an appropriate initial therapy.
Targeted therapy
Larotrectinib
Larotrectinib is an oral ATP-competitive inhibitor of TRK A, B, and C.
Evidence (larotrectinib):
- A phase I/II trial of larotrectinib was completed in patients with recurrent infantile fibrosarcoma who harbored an NTRK gene fusion.[95]
- Durable objective responses were seen in all eight patients, and responses occurred at a median of 1.7 months.
- Most toxicities were grades 1 and 2, which included transaminitis, leukopenia, neutropenia, and vomiting. There were no grade 4 or grade 5 events attributed to larotrectinib.
- In another study, three of five patients who achieved a partial response after neoadjuvant larotrectinib underwent a complete surgical resection with negative margins.[96,97]; [98][Level of evidence C2]
- These three patients achieved an excellent pathological response (>98% treatment effect) and remained disease free 7 to 15 months after surgery.
- In a follow-up report, 159 patients with TRK fusion–positive tumors were enrolled in three phase I/II trials. There were 28 patients with infantile fibrosarcoma who were treated with single-agent larotrectinib.[99][Level of evidence C2]
- The response rate was 96%.
- The Children’s Oncology Group conducted a phase II histology-agnostic trial of larotrectinib in children with NTRK fusion–positive solid tumors.[100] The study enrolled 33 patients, 18 with infantile fibrosarcoma and 15 with other solid tumors.
- The overall response rate within six cycles was 94% (17 of 18; 95% adjusted CI, 72.7%–98.6%) for children with infantile fibrosarcoma and 60% (9 of 15; 95% CI, 32.3%–83.7%) for children with other solid tumors.
- Six percent (2 of 33; 95% CI, 0.7%–22.2%) of patients developed progressive disease while on therapy.
- The 2-year EFS and OS rates among these groups were 82.2% (95% CI, 54.3%–93.9%) and 93.8% (95% CI, 63.2%–99.1%) for infantile fibrosarcoma and 80% (95% CI, 50.0%–93.1%) and 93.3% (95% CI, 61.3%–99.0%) for other solid tumors, respectively.
- Patients who underwent surgical resection of their tumor had prolonged EFS, with only 1 of these 16 patients experiencing disease progression.
Other TRK inhibitors
- LOXO-195: In a clinical trial, 1 of 8 pediatric patients with an ETV6::NTRK3–rearranged infantile fibrosarcoma developed progressive disease after 8 months of larotrectinib therapy and was found to have an acquired G623R resistance variant. The patient was treated with LOXO-195, a selective TRK inhibitor designed to overcome acquired resistance mediated by recurrent kinase domain variants. The patient experienced a transient partial response.[101] LOXO-195 is no longer being developed.
VEGFR inhibitor
- Pazopanib: A patient aged 2 months with infantile fibrosarcoma was initially treated with chemotherapy. At disease progression, a response was seen with pazopanib therapy.[102]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Fibrosarcoma NOS
These tumors lack the translocation seen in infantile fibrosarcomas. They present like most nonrhabdomyosarcomas, and the management approach is similar.
Myxofibrosarcoma
Myxofibrosarcoma is a rare lesion, especially in childhood. It is typically treated with complete surgical resection.
Low-Grade Fibromyxoid Sarcoma
Low-grade fibromyxoid sarcoma is a histologically deceptive soft tissue neoplasm that most commonly affects young and middle-aged adults. It is commonly located deep within the extremities.[103-105]
A Children's Oncology Group (COG) trial (ARST0332 [NCT00346164]) enrolled 11 patients with this tumor type. The median age at diagnosis was 13 years, and males were more commonly affected. The most common tumor sites were the lower and upper extremities (n = 9). None of the patients developed local or distant disease recurrence at a median follow up of 2.7 years.[106]
Genomic alterations
Low-grade fibromyxoid sarcoma is characterized by a FUS::CREB3L2 gene translocation and, rarely, alternative gene translocations such as FUS::CREB3L1 and EWSR1::CREB3L1.[107,108]
Prognosis
In a review of 33 patients (3 were younger than 18 years) with low-grade fibromyxoid sarcoma, 21 patients developed a local recurrence after intervals of up to 15 years (median, 3.5 years). Fifteen patients developed metastases up to 45 years (median, 5 years) from diagnosis, most commonly to the lungs and pleura. This finding emphasizes the need for continued follow-up of these patients.[103] Even after metastases occur, the disease course may be indolent.[109]
In another report, 14 of 73 patients were younger than 18 years. In this series with a relatively short follow-up (median of 24 months), only 8 of 54 patients with adequate follow-up developed local (9%) or distant (6%) disease recurrence. This report suggested that the behavior of this tumor might be significantly better than previously reported.[110] However, because late metastases can occur, careful monitoring of these patients is warranted.
A study by the Ultra-Rare Sarcoma Working Group examined 32 patients with rare occurrences of distant metastases (20 with metastases at diagnosis, 12 after initial resection). Most metastases occurred in the lungs. Treatments varied, and minimal responses were observed to anthracycline-based and gemcitabine-based regimens with trabectidin. However, there were few patients in each treatment group.[111]
Treatment of low-grade fibromyxoid sarcoma
Treatment options for low-grade fibromyxoid sarcoma include the following:
- Surgery.
Low-grade fibromyxoid sarcoma is not very chemosensitive, and the limited treatment information suggests that surgery is the treatment of choice.[109]
Evidence (surgery):
- The German Cooperative Weichteilsarkom Studiengruppe (CWS) reported study results for 31 patients younger than 21 years with low-grade fibromyxoid sarcoma.[104][Level of evidence C2]
- The 5-year EFS rate was 71% (95% CI, ±18.6%), the 5-year local relapse-free survival rate was 76% (95% CI, ±17.6%), and the 5-year OS rate was 100%.
- Among 24 patients who had R0 resections (complete resection with negative microscopic margins), 5 patients (21%) experienced disease relapses (3 local, 1 metastatic, and 1 combined).
- Among seven patients who had R1 resections (margins were microscopically positive), three patients (43%) experienced local disease relapses.
There are little data regarding the use of chemotherapy and/or radiation therapy in this disease. One report suggests that trabectedin may be effective in the treatment of low-grade fibromyxoid sarcoma.[112]
Sclerosing Epithelioid Fibrosarcoma
Sclerosing epithelioid fibrosarcoma is a rare malignant sarcoma that commonly harbors EWSR1 gene fusions and has an aggressive clinical course. The tumor responds poorly to chemotherapy.[113,114]
Genomic characteristics
Sclerosing epithelioid fibrosarcoma most commonly has the EWSR1::CREB3L1 gene fusion. However, EWSR1 may have other partners, including CREB3L2 and CREB3L3.[115,116] Gene fusions involving FUS (including the FUS::CREB3L2 fusion associated with low-grade fibromyxoid sarcoma) and PAX5 (e.g., PAX5::CREB3L1) are uncommon but can occur.[116,117] For cases of sclerosing epithelioid fibrosarcoma that lack MUC4 expression, EWSR1 gene fusions are generally absent, while a gene fusion involving YAP1 and KMT2A is commonly observed.[113,115,118,119] Sclerosing epithelioid fibrosarcoma has more structural and chromosomal segmental alterations than low-grade fibromyxoid fibrosarcoma.[115]
Treatment of sclerosing epithelioid fibrosarcoma
Treatment options for sclerosing epithelioid fibrosarcoma include the following:
- Surgery.
The tumor responds poorly to chemotherapy.[120] Therefore, it is typically treated with complete surgical excision. Long-term follow-up is recommended because late local recurrences and metastases can occur.
References
- Lewis JJ, Boland PJ, Leung DH, et al.: The enigma of desmoid tumors. Ann Surg 229 (6): 866-72; discussion 872-3, 1999. [PUBMED Abstract]
- Gounder MM, Mahoney MR, Van Tine BA, et al.: Sorafenib for Advanced and Refractory Desmoid Tumors. N Engl J Med 379 (25): 2417-2428, 2018. [PUBMED Abstract]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, et al.: A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 129 (1): 256-61, 2011. [PUBMED Abstract]
- Lazar AJ, Tuvin D, Hajibashi S, et al.: Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 173 (5): 1518-27, 2008. [PUBMED Abstract]
- Rossato M, Rigotti M, Grazia M, et al.: Congenital hypertrophy of the retinal pigment epithelium (CHRPE) and familial adenomatous polyposis (FAP). Acta Ophthalmol Scand 74 (4): 338-42, 1996. [PUBMED Abstract]
- Baker RH, Heinemann MH, Miller HH, et al.: Hyperpigmented lesions of the retinal pigment epithelium in familial adenomatous polyposis. Am J Med Genet 31 (2): 427-35, 1988. [PUBMED Abstract]
- Kattentidt Mouravieva AA, Geurts-Giele IR, de Krijger RR, et al.: Identification of Familial Adenomatous Polyposis carriers among children with desmoid tumours. Eur J Cancer 48 (12): 1867-74, 2012. [PUBMED Abstract]
- Wang WL, Nero C, Pappo A, et al.: CTNNB1 genotyping and APC screening in pediatric desmoid tumors: a proposed algorithm. Pediatr Dev Pathol 15 (5): 361-7, 2012 Sep-Oct. [PUBMED Abstract]
- Baday YI, Navai SA, Hicks MJ, et al.: Pediatric liposarcoma: A case series and literature review. Pediatr Blood Cancer 68 (12): e29327, 2021. [PUBMED Abstract]
- Merchant NB, Lewis JJ, Woodruff JM, et al.: Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 86 (10): 2045-52, 1999. [PUBMED Abstract]
- Faulkner LB, Hajdu SI, Kher U, et al.: Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol 13 (11): 2813-8, 1995. [PUBMED Abstract]
- Honeyman JN, Theilen TM, Knowles MA, et al.: Desmoid fibromatosis in children and adolescents: a conservative approach to management. J Pediatr Surg 48 (1): 62-6, 2013. [PUBMED Abstract]
- Bonvalot S, Ternès N, Fiore M, et al.: Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 20 (13): 4096-102, 2013. [PUBMED Abstract]
- Bonvalot S, Eldweny H, Haddad V, et al.: Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol 34 (4): 462-8, 2008. [PUBMED Abstract]
- Merchant TE, Nguyen D, Walter AW, et al.: Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys 47 (5): 1267-71, 2000. [PUBMED Abstract]
- Zelefsky MJ, Harrison LB, Shiu MH, et al.: Combined surgical resection and iridium 192 implantation for locally advanced and recurrent desmoid tumors. Cancer 67 (2): 380-4, 1991. [PUBMED Abstract]
- Weiss AJ, Lackman RD: Low-dose chemotherapy of desmoid tumors. Cancer 64 (6): 1192-4, 1989. [PUBMED Abstract]
- Klein WA, Miller HH, Anderson M, et al.: The use of indomethacin, sulindac, and tamoxifen for the treatment of desmoid tumors associated with familial polyposis. Cancer 60 (12): 2863-8, 1987. [PUBMED Abstract]
- Soto-Miranda MA, Sandoval JA, Rao B, et al.: Surgical treatment of pediatric desmoid tumors. A 12-year, single-center experience. Ann Surg Oncol 20 (11): 3384-90, 2013. [PUBMED Abstract]
- Desmoid Tumor Working Group: The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer 127: 96-107, 2020. [PUBMED Abstract]
- Penel N, Le Cesne A, Bonvalot S, et al.: Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer 83: 125-131, 2017. [PUBMED Abstract]
- Orbach D, Brennan B, Bisogno G, et al.: The EpSSG NRSTS 2005 treatment protocol for desmoid-type fibromatosis in children: an international prospective case series. Lancet Child Adolesc Health 1 (4): 284-292, 2017. [PUBMED Abstract]
- Skapek SX, Ferguson WS, Granowetter L, et al.: Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 25 (5): 501-6, 2007. [PUBMED Abstract]
- Gega M, Yanagi H, Yoshikawa R, et al.: Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol 24 (1): 102-5, 2006. [PUBMED Abstract]
- Constantinidou A, Jones RL, Scurr M, et al.: Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 45 (17): 2930-4, 2009. [PUBMED Abstract]
- Ananth P, Werger A, Voss S, et al.: Liposomal doxorubicin: Effective treatment for pediatric desmoid fibromatosis. Pediatr Blood Cancer 64 (7): , 2017. [PUBMED Abstract]
- Ferrari A, Orbach D, Affinita MC, et al.: Evidence of hydroxyurea activity in children with pretreated desmoid-type fibromatosis: A new option in the armamentarium of systemic therapies. Pediatr Blood Cancer 66 (1): e27472, 2019. [PUBMED Abstract]
- Kornreich L, Orbach D, Nicolas N, et al.: Oral vinorelbine in young patients with desmoid-type fibromatosis. Tumori 109 (5): 511-518, 2023. [PUBMED Abstract]
- Agresta L, Kim H, Turpin BK, et al.: Pazopanib therapy for desmoid tumors in adolescent and young adult patients. Pediatr Blood Cancer 65 (6): e26968, 2018. [PUBMED Abstract]
- Toulmonde M, Pulido M, Ray-Coquard I, et al.: Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): a non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol 20 (9): 1263-1272, 2019. [PUBMED Abstract]
- Shang H, Braggio D, Lee YJ, et al.: Targeting the Notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 121 (22): 4088-96, 2015. [PUBMED Abstract]
- Kummar S, O'Sullivan Coyne G, Do KT, et al.: Clinical Activity of the γ-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis). J Clin Oncol 35 (14): 1561-1569, 2017. [PUBMED Abstract]
- Gounder M, Ratan R, Alcindor T, et al.: Nirogacestat, a γ-Secretase Inhibitor for Desmoid Tumors. N Engl J Med 388 (10): 898-912, 2023. [PUBMED Abstract]
- Takahashi T, Prensner JR, Robson CD, et al.: Safety and efficacy of gamma-secretase inhibitor nirogacestat (PF-03084014) in desmoid tumor: Report of four pediatric/young adult cases. Pediatr Blood Cancer 67 (10): e28636, 2020. [PUBMED Abstract]
- Meazza C, Bisogno G, Gronchi A, et al.: Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer 116 (1): 233-40, 2010. [PUBMED Abstract]
- Hansmann A, Adolph C, Vogel T, et al.: High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 100 (3): 612-20, 2004. [PUBMED Abstract]
- Skapek SX, Anderson JR, Hill DA, et al.: Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children's Oncology Group (COG) phase II study. Pediatr Blood Cancer 60 (7): 1108-12, 2013. [PUBMED Abstract]
- Rutenberg MS, Indelicato DJ, Knapik JA, et al.: External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer 57 (3): 435-42, 2011. [PUBMED Abstract]
- Buckley PG, Mantripragada KK, Benetkiewicz M, et al.: A full-coverage, high-resolution human chromosome 22 genomic microarray for clinical and research applications. Hum Mol Genet 11 (25): 3221-9, 2002. [PUBMED Abstract]
- Valdivielso-Ramos M, Torrelo A, Campos M, et al.: Pediatric dermatofibrosarcoma protuberans in Madrid, Spain: multi-institutional outcomes. Pediatr Dermatol 31 (6): 676-82, 2014 Nov-Dec. [PUBMED Abstract]
- Gooskens SL, Oranje AP, van Adrichem LN, et al.: Imatinib mesylate for children with dermatofibrosarcoma protuberans (DFSP). Pediatr Blood Cancer 55 (2): 369-73, 2010. [PUBMED Abstract]
- Rubio GA, Alvarado A, Gerth DJ, et al.: Incidence and Outcomes of Dermatofibrosarcoma Protuberans in the US Pediatric Population. J Craniofac Surg 28 (1): 182-184, 2017. [PUBMED Abstract]
- Miller SJ, Alam M, Andersen JS, et al.: Dermatofibrosarcoma protuberans. J Natl Compr Canc Netw 10 (3): 312-8, 2012. [PUBMED Abstract]
- Meguerditchian AN, Wang J, Lema B, et al.: Wide excision or Mohs micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol 33 (3): 300-3, 2010. [PUBMED Abstract]
- Brennan B, Zanetti I, De Salvo GL, et al.: Dermatofibrosarcoma protuberans in children and adolescents: The European Paediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Pediatr Blood Cancer 67 (10): e28351, 2020. [PUBMED Abstract]
- Dagan R, Morris CG, Zlotecki RA, et al.: Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am J Clin Oncol 28 (6): 537-9, 2005. [PUBMED Abstract]
- Sun LM, Wang CJ, Huang CC, et al.: Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol 57 (2): 175-81, 2000. [PUBMED Abstract]
- Price VE, Fletcher JA, Zielenska M, et al.: Imatinib mesylate: an attractive alternative in young children with large, surgically challenging dermatofibrosarcoma protuberans. Pediatr Blood Cancer 44 (5): 511-5, 2005. [PUBMED Abstract]
- McArthur GA, Demetri GD, van Oosterom A, et al.: Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol 23 (4): 866-73, 2005. [PUBMED Abstract]
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al.: Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 28 (10): 1772-9, 2010. [PUBMED Abstract]
- Navarrete-Dechent C, Mori S, Barker CA, et al.: Imatinib Treatment for Locally Advanced or Metastatic Dermatofibrosarcoma Protuberans: A Systematic Review. JAMA Dermatol 155 (3): 361-369, 2019. [PUBMED Abstract]
- Kovach SJ, Fischer AC, Katzman PJ, et al.: Inflammatory myofibroblastic tumors. J Surg Oncol 94 (5): 385-91, 2006. [PUBMED Abstract]
- Brodlie M, Barwick SC, Wood KM, et al.: Inflammatory myofibroblastic tumours of the respiratory tract: paediatric case series with varying clinical presentations. J Laryngol Otol 125 (8): 865-8, 2011. [PUBMED Abstract]
- Xiao Y, Zhou S, Ma C, et al.: Radiological and histopathological features of hepatic inflammatory myofibroblastic tumour: analysis of 10 cases. Clin Radiol 68 (11): 1114-20, 2013. [PUBMED Abstract]
- Karnak I, Senocak ME, Ciftci AO, et al.: Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 36 (6): 908-12, 2001. [PUBMED Abstract]
- Collin M, Charles A, Barker A, et al.: Inflammatory myofibroblastic tumour of the bladder in children: a review. J Pediatr Urol 11 (5): 239-45, 2015. [PUBMED Abstract]
- Mariño-Enríquez A, Wang WL, Roy A, et al.: Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol 35 (1): 135-44, 2011. [PUBMED Abstract]
- Lee JC, Li CF, Huang HY, et al.: ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol 241 (3): 316-323, 2017. [PUBMED Abstract]
- Trahair T, Gifford AJ, Fordham A, et al.: Crizotinib and Surgery for Long-Term Disease Control in Children and Adolescents With ALK-Positive Inflammatory Myofibroblastic Tumors. JCO Precis Oncol 3: , 2019. [PUBMED Abstract]
- Coffin CM, Hornick JL, Fletcher CD: Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31 (4): 509-20, 2007. [PUBMED Abstract]
- Lovly CM, Gupta A, Lipson D, et al.: Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4 (8): 889-95, 2014. [PUBMED Abstract]
- Casanova M, Brennan B, Alaggio R, et al.: Inflammatory myofibroblastic tumor: The experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur J Cancer 127: 123-129, 2020. [PUBMED Abstract]
- Yu L, Liu J, Lao IW, et al.: Epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological, immunohistochemical and molecular cytogenetic analysis of five additional cases and review of the literature. Diagn Pathol 11 (1): 67, 2016. [PUBMED Abstract]
- Devaney KO, Lafeir DJ, Triantafyllou A, et al.: Inflammatory myofibroblastic tumors of the head and neck: evaluation of clinicopathologic and prognostic features. Eur Arch Otorhinolaryngol 269 (12): 2461-5, 2012. [PUBMED Abstract]
- Mehta B, Mascarenhas L, Zhou S, et al.: Inflammatory myofibroblastic tumors in childhood. Pediatr Hematol Oncol 30 (7): 640-5, 2013. [PUBMED Abstract]
- Kube S, Vokuhl C, Dantonello T, et al.: Inflammatory myofibroblastic tumors-A retrospective analysis of the Cooperative Weichteilsarkom Studiengruppe. Pediatr Blood Cancer 65 (6): e27012, 2018. [PUBMED Abstract]
- Dalton BG, Thomas PG, Sharp NE, et al.: Inflammatory myofibroblastic tumors in children. J Pediatr Surg 51 (4): 541-4, 2016. [PUBMED Abstract]
- Favini F, Resti AG, Collini P, et al.: Inflammatory myofibroblastic tumor of the conjunctiva: response to chemotherapy with low-dose methotrexate and vinorelbine. Pediatr Blood Cancer 54 (3): 483-5, 2010. [PUBMED Abstract]
- Schoot RA, Orbach D, Minard Colin V, et al.: Inflammatory Myofibroblastic Tumor With ROS1 Gene Fusions in Children and Young Adolescents. JCO Precis Oncol 7: e2300323, 2023. [PUBMED Abstract]
- Doski JJ, Priebe CJ, Driessnack M, et al.: Corticosteroids in the management of unresected plasma cell granuloma (inflammatory pseudotumor) of the lung. J Pediatr Surg 26 (9): 1064-6, 1991. [PUBMED Abstract]
- Diop B, Konate I, Ka S, et al.: Mesenteric myofibroblastic tumor: NSAID therapy after incomplete resection. J Visc Surg 148 (4): e311-4, 2011. [PUBMED Abstract]
- Mossé YP, Lim MS, Voss SD, et al.: Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 14 (6): 472-80, 2013. [PUBMED Abstract]
- Gaudichon J, Jeanne-Pasquier C, Deparis M, et al.: Complete and Repeated Response of a Metastatic ALK-rearranged Inflammatory Myofibroblastic Tumor to Crizotinib in a Teenage Girl. J Pediatr Hematol Oncol 38 (4): 308-11, 2016. [PUBMED Abstract]
- Mossé YP, Voss SD, Lim MS, et al.: Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol 35 (28): 3215-3221, 2017. [PUBMED Abstract]
- Butrynski JE, D'Adamo DR, Hornick JL, et al.: Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363 (18): 1727-33, 2010. [PUBMED Abstract]
- Nakano K: Inflammatory myofibroblastic tumors: recent progress and future of targeted therapy. Jpn J Clin Oncol 53 (10): 885-892, 2023. [PUBMED Abstract]
- Brivio E, Zwaan CM: ALK inhibition in two emblematic cases of pediatric inflammatory myofibroblastic tumor: Efficacy and side effects. Pediatr Blood Cancer 66 (5): e27645, 2019. [PUBMED Abstract]
- Fischer M, Moreno L, Ziegler DS, et al.: Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol 22 (12): 1764-1776, 2021. [PUBMED Abstract]
- Nishio M, Murakami H, Horiike A, et al.: Phase I Study of Ceritinib (LDK378) in Japanese Patients with Advanced, Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer or Other Tumors. J Thorac Oncol 10 (7): 1058-66, 2015. [PUBMED Abstract]
- Fujiki T, Sakai Y, Ikawa Y, et al.: Pediatric inflammatory myofibroblastic tumor of the bladder with ALK-FN1 fusion successfully treated by alectinib. Pediatr Blood Cancer 70 (4): e30172, 2023. [PUBMED Abstract]
- Gounder MM, Agaram NP, Trabucco SE, et al.: Clinical genomic profiling in the management of patients with soft tissue and bone sarcoma. Nat Commun 13 (1): 3406, 2022. [PUBMED Abstract]
- Sulkowski JP, Raval MV, Browne M: Margin status and multimodal therapy in infantile fibrosarcoma. Pediatr Surg Int 29 (8): 771-6, 2013. [PUBMED Abstract]
- Hirschfeld R, Welch JJG, Harrison DJ, et al.: Two cases of humoral hypercalcemia of malignancy complicating infantile fibrosarcoma. Pediatr Blood Cancer 64 (10): , 2017. [PUBMED Abstract]
- Kao YC, Fletcher CDM, Alaggio R, et al.: Recurrent BRAF Gene Fusions in a Subset of Pediatric Spindle Cell Sarcomas: Expanding the Genetic Spectrum of Tumors With Overlapping Features With Infantile Fibrosarcoma. Am J Surg Pathol 42 (1): 28-38, 2018. [PUBMED Abstract]
- Wegert J, Vokuhl C, Collord G, et al.: Recurrent intragenic rearrangements of EGFR and BRAF in soft tissue tumors of infants. Nat Commun 9 (1): 2378, 2018. [PUBMED Abstract]
- Tan SY, Al-Ibraheemi A, Ahrens WA, et al.: ALK rearrangements in infantile fibrosarcoma-like spindle cell tumours of soft tissue and kidney. Histopathology 80 (4): 698-707, 2022. [PUBMED Abstract]
- Motta M, Barresi S, Pizzi S, et al.: RAF1 gene fusions are recurrent driver events in infantile fibrosarcoma-like mesenchymal tumors. J Pathol 263 (2): 166-177, 2024. [PUBMED Abstract]
- Orbach D, Rey A, Cecchetto G, et al.: Infantile fibrosarcoma: management based on the European experience. J Clin Oncol 28 (2): 318-23, 2010. [PUBMED Abstract]
- Orbach D, Brennan B, De Paoli A, et al.: Conservative strategy in infantile fibrosarcoma is possible: The European paediatric Soft tissue sarcoma Study Group experience. Eur J Cancer 57: 1-9, 2016. [PUBMED Abstract]
- Hawkins DS, Black JO, Orbach D, et al.: Nonrhabdomyosarcoma soft-tissue sarcomas. In: Blaney SM, Helman LJ, Adamson PC, eds.: Pizzo and Poplack's Pediatric Oncology. 8th ed. Wolters Kluwer, 2020, pp 721-46.
- Loh ML, Ahn P, Perez-Atayde AR, et al.: Treatment of infantile fibrosarcoma with chemotherapy and surgery: results from the Dana-Farber Cancer Institute and Children's Hospital, Boston. J Pediatr Hematol Oncol 24 (9): 722-6, 2002. [PUBMED Abstract]
- Akyüz C, Küpeli S, Varan A, et al.: Infantile fibrosarcoma: retrospective analysis of eleven patients. Tumori 97 (2): 166-9, 2011 Mar-Apr. [PUBMED Abstract]
- Gallego S, Pericas N, Barber I, et al.: Infantile fibrosarcoma of the retroperitoneum: a site of unfavorable prognosis? Pediatr Hematol Oncol 28 (5): 451-3, 2011. [PUBMED Abstract]
- Parida L, Fernandez-Pineda I, Uffman JK, et al.: Clinical management of infantile fibrosarcoma: a retrospective single-institution review. Pediatr Surg Int 29 (7): 703-8, 2013. [PUBMED Abstract]
- Laetsch TW, DuBois SG, Mascarenhas L, et al.: Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 19 (5): 705-714, 2018. [PUBMED Abstract]
- Kummar S, Lassen UN: TRK Inhibition: A New Tumor-Agnostic Treatment Strategy. Target Oncol 13 (5): 545-556, 2018. [PUBMED Abstract]
- Drilon A, Laetsch TW, Kummar S, et al.: Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378 (8): 731-739, 2018. [PUBMED Abstract]
- DuBois SG, Laetsch TW, Federman N, et al.: The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer 124 (21): 4241-4247, 2018. [PUBMED Abstract]
- Hong DS, DuBois SG, Kummar S, et al.: Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21 (4): 531-540, 2020. [PUBMED Abstract]
- Laetsch TW, Voss S, Ludwig K, et al.: Larotrectinib for Newly Diagnosed Infantile Fibrosarcoma and Other Pediatric NTRK Fusion-Positive Solid Tumors (Children's Oncology Group ADVL1823). J Clin Oncol 43 (10): 1188-1197, 2025. [PUBMED Abstract]
- Drilon A, Nagasubramanian R, Blake JF, et al.: A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov 7 (9): 963-972, 2017. [PUBMED Abstract]
- Yanagisawa R, Noguchi M, Fujita K, et al.: Preoperative Treatment With Pazopanib in a Case of Chemotherapy-Resistant Infantile Fibrosarcoma. Pediatr Blood Cancer 63 (2): 348-51, 2016. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Scheer M, Vokuhl C, Veit-Friedrich I, et al.: Low-grade fibromyxoid sarcoma: A report of the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer 67 (2): e28009, 2020. [PUBMED Abstract]
- Fletcher CDM, Bridge JA, Hogendoorn P, et al., eds.: WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press, 2013.
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Guillou L, Benhattar J, Gengler C, et al.: Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol 31 (9): 1387-402, 2007. [PUBMED Abstract]
- Mohamed M, Fisher C, Thway K: Low-grade fibromyxoid sarcoma: Clinical, morphologic and genetic features. Ann Diagn Pathol 28: 60-67, 2017. [PUBMED Abstract]
- O'Sullivan MJ, Sirgi KE, Dehner LP: Low-grade fibrosarcoma (hyalinizing spindle cell tumor with giant rosettes) with pulmonary metastases at presentation: case report and review of the literature. Int J Surg Pathol 10 (3): 211-6, 2002. [PUBMED Abstract]
- Folpe AL, Lane KL, Paull G, et al.: Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol 24 (10): 1353-60, 2000. [PUBMED Abstract]
- Giani C, Denu RA, Ljevar S, et al.: Low-grade fibromyxoid sarcoma and sclerosing epithelioid fibrosarcoma, outcome of advanced disease: retrospective study from the Ultra-Rare Sarcoma Working Group. ESMO Open 9 (9): 103689, 2024. [PUBMED Abstract]
- Maretty-Nielsen K, Baerentzen S, Keller J, et al.: Low-Grade Fibromyxoid Sarcoma: Incidence, Treatment Strategy of Metastases, and Clinical Significance of the FUS Gene. Sarcoma 2013: 256280, 2013. [PUBMED Abstract]
- Prieto-Granada C, Zhang L, Chen HW, et al.: A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer 54 (1): 28-38, 2015. [PUBMED Abstract]
- Arbajian E, Puls F, Antonescu CR, et al.: In-depth Genetic Analysis of Sclerosing Epithelioid Fibrosarcoma Reveals Recurrent Genomic Alterations and Potential Treatment Targets. Clin Cancer Res 23 (23): 7426-7434, 2017. [PUBMED Abstract]
- Arbajian E, Puls F, Magnusson L, et al.: Recurrent EWSR1-CREB3L1 gene fusions in sclerosing epithelioid fibrosarcoma. Am J Surg Pathol 38 (6): 801-8, 2014. [PUBMED Abstract]
- Dewaele B, Libbrecht L, Levy G, et al.: A novel EWS-CREB3L3 gene fusion in a mesenteric sclerosing epithelioid fibrosarcoma. Genes Chromosomes Cancer 56 (9): 695-699, 2017. [PUBMED Abstract]
- Porteus C, Gan Q, Gong Y, et al.: Sclerosing epithelioid fibrosarcoma: cytologic characterization with histologic, immunohistologic, molecular, and clinical correlation of 8 cases. J Am Soc Cytopathol 9 (6): 513-519, 2020 Nov - Dec. [PUBMED Abstract]
- Puls F, Agaimy A, Flucke U, et al.: Recurrent Fusions Between YAP1 and KMT2A in Morphologically Distinct Neoplasms Within the Spectrum of Low-grade Fibromyxoid Sarcoma and Sclerosing Epithelioid Fibrosarcoma. Am J Surg Pathol 44 (5): 594-606, 2020. [PUBMED Abstract]
- Warmke LM, Meis JM: Sclerosing Epithelioid Fibrosarcoma: A Distinct Sarcoma With Aggressive Features. Am J Surg Pathol 45 (3): 317-328, 2021. [PUBMED Abstract]
- Chew W, Benson C, Thway K, et al.: Clinical Characteristics and efficacy of chemotherapy in sclerosing epithelioid fibrosarcoma. Med Oncol 35 (11): 138, 2018. [PUBMED Abstract]
Treatment of Skeletal Muscle Tumors
Skeletal muscle tumors have several subtypes, including the following:
Rhabdomyosarcoma
For more information, see Childhood Rhabdomyosarcoma Treatment.
Ectomesenchymoma
Ectomesenchymoma is a rare skeletal muscle tumor that mainly occurs in children. It is a biphenotypic soft tissue sarcoma with both mesenchymal and ectodermal components.
A single-institution retrospective review identified seven cases of malignant ectomesenchymoma.[1] All seven patients were male, with a mean age of 7.5 months (range, 0.6–17.0 months). Five of the seven patients in this series were healthy and free of disease at the time of reporting.
Histological features and genomic alterations
A retrospective review of six patients with malignant ectomesenchymoma from a single institution identified rhabdomyosarcoma as the mesenchymal element in five of six tumors.[2] Tumors with an alveolar rhabdomyosarcoma morphology exhibited the characteristic translocations, including translocation of the FOXO1 gene fusing with the PAX3 or PAX7 gene. No unifying molecular aberrations were identified.
A single-institution retrospective review identified seven cases of malignant ectomesenchymoma.[1] Most patients showed elements of embryonal rhabdomyosarcoma. The mixed neuroectodermal elements were scattered ganglion cells, ganglioneuroma, or ganglioneuroblastoma. Six of seven cases had HRAS variants. The trimethylation at lysine 27 of histone H3 (H3K27me3), typically lost in malignant peripheral nerve sheath tumor, was retained in all cases.
Treatment of ectomesenchymoma
Treatment options for ectomesenchymoma include the following:
- Surgery.
- Chemotherapy.
- Radiation therapy.
The Cooperative Weichteilsarkom Studiengruppe (CWS) reported on six patients (aged 0.2–13.5 years) registered over 14 years.[3][Level of evidence C1] The tumors were located in various sites, including the extremities, abdomen, and orbit. All six patients were treated with surgery and chemotherapy directed at rhabdomyosarcoma. Two patients received radiation therapy. Three patients experienced tumor recurrences with rhabdomyosarcoma features. Although data are scant, it appears that the tumor may respond to chemotherapy.[3]
The European paediatric Soft Tissue Sarcoma Study Group (EpSSG) identified ten patients with ectomesenchymoma in a prospectively recorded database.[4] Of the ten cases, seven had an initial local diagnosis of rhabdomyosarcoma. All patients received chemotherapy according to rhabdomyosarcoma strategy, and four patients received radiation therapy. Overall, six patients were alive in first remission, two in second remission, and one after treatment for a second primary cancer. Only the patient with a metastatic tumor at diagnosis died of their disease.
References
- Huang SC, Alaggio R, Sung YS, et al.: Frequent HRAS Mutations in Malignant Ectomesenchymoma: Overlapping Genetic Abnormalities With Embryonal Rhabdomyosarcoma. Am J Surg Pathol 40 (7): 876-85, 2016. [PUBMED Abstract]
- Griffin BB, Chou PM, George D, et al.: Malignant Ectomesenchymoma: Series Analysis of a Histologically and Genetically Heterogeneous Tumor. Int J Surg Pathol 26 (3): 200-212, 2018. [PUBMED Abstract]
- Dantonello TM, Leuschner I, Vokuhl C, et al.: Malignant ectomesenchymoma in children and adolescents: report from the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer 60 (2): 224-9, 2013. [PUBMED Abstract]
- Milano GM, Orbach D, Casanova M, et al.: Malignant ectomesenchymoma in children: The European pediatric Soft tissue sarcoma Study Group experience. Pediatr Blood Cancer 70 (2): e30116, 2023. [PUBMED Abstract]
Treatment of Smooth Muscle Tumors
Leiomyosarcoma Not Otherwise Specified (NOS)
Leiomyosarcoma accounts for 2% of soft tissue sarcomas in patients younger than 20 years (see Table 1).
Risk factors
Among 43 children with HIV/AIDS who developed tumors, 8 developed Epstein-Barr virus–associated leiomyosarcoma.[1] Survivors of hereditary retinoblastoma have a statistically significant increased risk of developing leiomyosarcoma, and 78% of these patients were diagnosed 30 or more years after the initial diagnosis of retinoblastoma.[2]
Treatment of leiomyosarcoma
There are no standard treatment options for leiomyosarcoma in pediatric patients.
Trabectedin, an alkylating drug with multiple mechanisms of action that damage DNA, has been studied in adults with leiomyosarcoma. There are no studies using trabectedin to treat leiomyosarcoma in pediatric patients.
Results from studies in adult patients include the following:
- In an open-label study of trabectedin in adult patients with recurrent sarcomas, the best overall response rate (complete remission and partial remission) was seen in patients with leiomyosarcoma (7.5%).[3] The clinical benefit rate (included stable disease) was 54% for patients with leiomyosarcoma.
- In another adult study, patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to receive treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[4]
- The French Sarcoma group conducted a randomized trial for the treatment of adult patients with leiomyosarcoma (age range, 52–69 years).[5] Patients were randomly assigned to receive either single-agent doxorubicin (six cycles) or doxorubicin with trabectedin, with continued maintenance therapy using trabectedin for patients in the doxorubicin/trabectedin group who did not have disease progression. Surgery to resect residual disease was allowed in each group after six cycles of therapy. The median overall survival was longer in the doxorubicin/trabectedin group (33 months; 95% confidence interval [CI], 26–48) than in the doxorubicin group (24 months; 95% CI, 19–31). The adjusted hazard ratio for death was 0.65 (95% CI, 0.44–0.95).
References
- Pollock BH, Jenson HB, Leach CT, et al.: Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA 289 (18): 2393-9, 2003. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Samuels BL, Chawla S, Patel S, et al.: Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 24 (6): 1703-9, 2013. [PUBMED Abstract]
- Demetri GD, von Mehren M, Jones RL, et al.: Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 34 (8): 786-93, 2016. [PUBMED Abstract]
- Pautier P, Italiano A, Piperno-Neumann S, et al.: Doxorubicin-Trabectedin with Trabectedin Maintenance in Leiomyosarcoma. N Engl J Med 391 (9): 789-799, 2024. [PUBMED Abstract]
Treatment of So-Called Fibrohistiocytic Tumors
Plexiform Fibrohistiocytic Tumor
Plexiform fibrohistiocytic tumor is a rare, low- to intermediate-grade so-called fibrohistiocytic tumor that most commonly affects children and young adults. The median age at presentation ranges from 8 to 14.5 years. However, the tumor has been described in patients as young as 3 months.[1,2]
Clinical presentation
The tumor commonly arises as a painless mass in the skin or subcutaneous tissue and most often involves the upper extremities, including the fingers, hand, and wrist.[3-5] Plexiform fibrohistiocytic tumor is an intermediate-grade tumor that rarely metastasizes. However, there are rare reports of the tumor spreading to regional lymph nodes or the lungs.[1,5,6]
Genomic alterations
No consistent chromosomal anomalies have been detected, but a t(4;15)(q21;q15) translocation has been reported.[7]
Treatment of plexiform fibrohistiocytic tumor
Treatment options for plexiform fibrohistiocytic tumor include the following:
- Surgery.
Surgery is the treatment of choice, but local recurrence has been reported in 12% to 50% of cases.[8]
References
- Enzinger FM, Zhang RY: Plexiform fibrohistiocytic tumor presenting in children and young adults. An analysis of 65 cases. Am J Surg Pathol 12 (11): 818-26, 1988. [PUBMED Abstract]
- Black J, Coffin CM, Dehner LP: Fibrohistiocytic tumors and related neoplasms in children and adolescents. Pediatr Dev Pathol 15 (1 Suppl): 181-210, 2012. [PUBMED Abstract]
- Moosavi C, Jha P, Fanburg-Smith JC: An update on plexiform fibrohistiocytic tumor and addition of 66 new cases from the Armed Forces Institute of Pathology, in honor of Franz M. Enzinger, MD. Ann Diagn Pathol 11 (5): 313-9, 2007. [PUBMED Abstract]
- Billings SD, Folpe AL: Cutaneous and subcutaneous fibrohistiocytic tumors of intermediate malignancy: an update. Am J Dermatopathol 26 (2): 141-55, 2004. [PUBMED Abstract]
- Remstein ED, Arndt CA, Nascimento AG: Plexiform fibrohistiocytic tumor: clinicopathologic analysis of 22 cases. Am J Surg Pathol 23 (6): 662-70, 1999. [PUBMED Abstract]
- Salomao DR, Nascimento AG: Plexiform fibrohistiocytic tumor with systemic metastases: a case report. Am J Surg Pathol 21 (4): 469-76, 1997. [PUBMED Abstract]
- Redlich GC, Montgomery KD, Allgood GA, et al.: Plexiform fibrohistiocytic tumor with a clonal cytogenetic anomaly. Cancer Genet Cytogenet 108 (2): 141-3, 1999. [PUBMED Abstract]
- Luzar B, Calonje E: Cutaneous fibrohistiocytic tumours - an update. Histopathology 56 (1): 148-65, 2010. [PUBMED Abstract]
Treatment of Peripheral Nerve Sheath Tumors
Peripheral nerve sheath tumors have several subtypes, including the following:
Malignant Peripheral Nerve Sheath Tumor (MPNST) NOS
MPNSTs account for 5% of soft tissue sarcomas in patients younger than 20 years (see Table 1).
Risk factors
MPNST can arise sporadically and in children with neurofibromatosis type 1 (NF1).[1] Among patients with NF1, a family history of MPNST is associated with an increased risk of developing early-onset MPNST.[2]
A rare case of a child with documented neurofibromatosis type 2 (NF2) and a benign neurofibroma had five recurrences of disease. During this time, the lesions progressively lost markers (such as S-100) and acquired clear-cut signs of malignant transformation to MPNST, documented by multiple markers, including the first example of NOTCH2 in this disease.[3]
Histological features, diagnostic evaluation, and genomic alterations
The molecular pathogenesis of adult MPNSTs demonstrates inactivating variants in at least three pathways, including NF1, CDKN2A, CDKN2B, and PRC2 complex core components. Similar alterations have been reported in pediatric tumors.[4]
- Inactivating variants of SUZ12 have been described in these tumors and are absent in neurofibromas.[5]
- A DNA methylation array for methylome-based and profile-based chromosomal characterization was performed on 171 peripheral nerve sheath tumors.[6] Atypical neurofibromas and low-grade MPNSTs were indistinguishable, with a common methylation profile and loss of CDKN2A. Epigenomic analysis identified two groups of conventional high-grade MPNSTs sharing a frequent loss of neurofibromin. The larger group showed an additional loss of trimethylation of H3K27me3. The smaller group of patients with predominantly spinal primary sites showed retention of H3K27me3.
- Genomic profiling was performed on 201 MPNSTs.[7] Thirteen of 201 tumors demonstrated BRAF alterations.
The Memorial Sloan Kettering Cancer Center studied archival and consultation material from 64 pediatric and young adult patients (aged 20 years or younger).[4] Fifty-eight percent of patients had a clinical history of NF1. All but one patient had high-grade MPNSTs. Overall, 89% of patients were classified as having high-grade MPNSTs, and 94% of patients had conventional histological features. There were 16 high-grade tumors available for molecular characterization using the MSK-IMPACT assay. These pediatric and adolescent tumors had genomic driver events that were similar to those in adult tumors. The study found genomic perturbations in PRC2 components (SUZ12 or EED; 9 cases), NF1 variants (8 cases), and CDKN2A and CDKN2B deletions (8 cases). Loss of HDK27me3 expression was noted in 82% of conventional high-grade MPNSTs. This finding is a potentially powerful immunohistochemical diagnostic marker for pediatric MPNSTs.
Prognostic factors and prognosis
Factors associated with a favorable prognosis include the following:[1,8-10]
Factors associated with an unfavorable prognosis include the following:[12]
- High grade.
- Deep tumor location.
- Locally advanced stage at diagnosis.
- Presence of metastasis at diagnosis. A retrospective review of 140 patients with MPNST from the MD Anderson Cancer Center included children and adolescents. The disease-specific survival at 10 years was 32%. In this series, presence of metastatic disease was associated with a much worse prognosis.[9]
- Macroscopically incomplete resection (R2).
- Inactivation of TP53, either by variant or amplification of MDM2.[13]
- High expression of TP53 and cyclin D1. These markers were identified as adverse prognostic factors using immunohistochemical staining of diagnostic biopsy tissue.[14][Level of evidence C2]
Presence of NF1 appears to be associated with an unfavorable prognosis, but the data are mixed.[4,15]
For patients with localized disease in the MD Anderson Cancer Center study, there was no significant difference in outcome between patients with and without NF1.[9] In other studies, it was not clear whether the absence of NF1 was a favorable prognostic factor as it has been associated with both favorable [8] and unfavorable outcomes.[1,8,10]
In the French Sarcoma Group study, NF1 was associated with other adverse prognostic features, but was not an independent predictor of poor outcome.[12] A retrospective analysis of cancer registry data from the Netherlands identified 784 patients with MPNST; 70 of the patients were aged 18 years or younger.[16][Level of evidence C1] In children with NF1, large tumor size was more common (>5 cm, 92.3% vs. 59.1%). Overall, the estimated 5-year survival rate was 52.4% (standard error [SE], 10.1%) for patients with localized MPNST and NF1, compared with 75.8% (SE, 7.1%) for patients without NF1.
The Cooperative Weichteilsarkom Studiengruppe (CWS) reported a retrospective review of patients with MPNST who were treated on five consecutive CWS trials.[17] A total of 159 patients were analyzed. NF1 was reported in 38 patients (24%). Nodal involvement was documented in 15 patients (9%) at diagnosis, and distant metastases was noted in 15 patients (9%) at diagnosis. Overall, the event-free survival (EFS) rate was 40.5% at 5 years and 36.3% at 10 years. The overall survival (OS) rate was 54.6% at 5 years and 47.1% at 10 years. Older age, positive NF1 status, primary tumor site other than extremity, larger tumor size, higher Intergroup Rhabdomyosarcoma Study (IRS) group, presence of metastatic disease, and failure to achieve first complete remission were identified as adverse prognostic factors for EFS and/or OS in the univariate analysis.
Treatment of MPNST
Treatment options for MPNST include the following:
- Surgery preceded or followed by radiation therapy.[18,19]
- Chemotherapy, for unresectable tumors.
Surgery preceded or followed by radiation therapy
Complete surgical removal of the tumor, whenever possible, is the mainstay of treatment.
The role of radiation therapy is difficult to assess, but durable local control of known postoperative microscopic residual tumor is not ensured after radiation therapy.
Chemotherapy
Chemotherapy has achieved objective responses in childhood MPNST.
Evidence (chemotherapy):
- A large retrospective analysis of the German and Italian experience with this tumor reported the following results:[1]
- Sixty-five percent of measurable tumors had objective responses to ifosfamide-containing chemotherapy regimens.
- The analysis did not conclusively demonstrate improved survival with chemotherapy.
- This retrospective analysis also noted a trend toward improved outcome with postoperative radiation therapy.
- A series of 37 young patients with MPNST and NF1 showed that most patients had large invasive tumors that were poorly responsive to chemotherapy.[20]
- The progression-free survival (PFS) rate was 19%, and the 5-year OS rate was 28%.
- The European paediatric Soft Tissue Sarcoma Study Group (EpSSG) performed a prospective study in patients aged 21 years and younger with MPNST.[21] Surgical resection of primary tumors was classified as R0 if the resection was complete with negative microscopic margins, R1 if the margins were microscopically positive, and R2 if the resection left macroscopic residual tumor. Patients were nonrandomly assigned to one of the following four treatment groups:
- Cohort 1: Patients with completely resected tumors (R0) who received surgery only (n = 13); the 5-year EFS rate was 92%.
- Cohort 2: Patients with incompletely resected tumors (R1/R2) who received adjuvant radiation therapy (n = 4); the 5-year EFS rate was 33%.
- Cohort 3: Patients with incompletely resected tumors (R1/R2) who received adjuvant chemotherapy (n = 7); the 5-year EFS rate was 29%.
- Cohort 4: Patients who received either chemotherapy before surgical resection and/or who had nodal involvement (n = 27); the 5-year EFS rate was 52%.
For patients who received chemotherapy, treatment consisted of four courses of ifosfamide/doxorubicin and two courses of ifosfamide concomitant with radiation therapy (50.4–54 Gy).
- The response rate to chemotherapy (partial response + complete response) in patients with measurable disease was 46%.
- The presence of NF1 (51% of patients) was an independent poor prognostic factor for OS and EFS.
- In a study of pediatric and adult patients with either sporadic (n = 14) or chemotherapy-naïve, NF1-associated (n = 34) MPNST, patients were treated with two cycles of ifosfamide and doxorubicin and two cycles of ifosfamide and etoposide.[22]
- Response rates were lower in patients with NF1-associated tumors than in patients with sporadic tumors (17.9% vs. 44.4%). However, the premature closure of the study did not allow sufficient power to detect meaningful differences in objective responses between the two groups.
- The rates of stable disease were similar between the two groups.
Recurrent MPNST
Of 120 patients enrolled in Italian pediatric protocols from 1979 to 2004, an analysis identified 73 patients younger than 21 years with relapsed MPNST. Treatment options included surgery, radiation therapy, and chemotherapy.[23]
- The time to relapse from initial diagnosis ranged from 1 month to 204 months, with a median time to relapse of 7 months.
- Median OS from first relapse was 11 months, with an OS rate of 39% at 1 year and 16% at 5 years.
- The factors associated with a higher probability of survival after relapse were lower tumor invasiveness at initial presentation, longer time to relapse, and complete surgical resection of the tumor at relapse.
A retrospective study evaluated nine patients with unresectable or metastatic MPNST (seven patients were previously treated) who were treated with selinexor with or without doxorubicin. Three patients experienced a partial response that lasted for 3 months to longer than 8 months, and four patients had stable disease.[24]
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT04465643 (Neoadjuvant Nivolumab Plus Ipilimumab for Newly Diagnosed Malignant Peripheral Nerve Sheath Tumor): The purpose of the study is to evaluate the safety and feasibility of neoadjuvant nivolumab plus ipilimumab before standard therapy (surgery, chemotherapy, or radiation therapy) in patients with NF1 and newly diagnosed premalignant and malignant peripheral nerve sheath tumors for whom surgery for resection of tumor is indicated.
Malignant Triton Tumor
Malignant triton tumors are now classified as a variant of MPNSTs. They occur most often in patients with NF1 and consist of neurogenic and rhabdomyoblastic components.[25] Most malignant triton tumors are reported in adults, although they may also arise in children and adolescents.[26]
Distinguishing between malignant triton tumors and NF1-altered rhabdomyosarcomas can be difficult. The genomic characteristics of malignant triton tumors can aid in differentiating between the two tumors. CDKN2A deep deletions and loss-of-function alterations in genes of the PRC2 complex (e.g., SUZ12 and EED1) are commonly observed in malignant triton tumors, while they are uncommon in NF1-altered rhabdomyosarcomas. The loss of PRC2 function leads to loss of H3K27me3 expression, a finding that is common in malignant triton tumors. H3K27me3 expression is generally maintained in rhabdomyosarcomas.[26-28]
References
- Carli M, Ferrari A, Mattke A, et al.: Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 23 (33): 8422-30, 2005. [PUBMED Abstract]
- Malbari F, Spira M, B Knight P, et al.: Malignant Peripheral Nerve Sheath Tumors in Neurofibromatosis: Impact of Family History. J Pediatr Hematol Oncol 40 (6): e359-e363, 2018. [PUBMED Abstract]
- Agresta L, Salloum R, Hummel TR, et al.: Malignant peripheral nerve sheath tumor: Transformation in a patient with neurofibromatosis type 2. Pediatr Blood Cancer 66 (2): e27520, 2019. [PUBMED Abstract]
- Agaram NP, Wexler LH, Chi P, et al.: Malignant peripheral nerve sheath tumor in children: A clinicopathologic and molecular study with parallels to the adult counterpart. Genes Chromosomes Cancer 62 (3): 131-138, 2023. [PUBMED Abstract]
- Zhang M, Wang Y, Jones S, et al.: Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 46 (11): 1170-2, 2014. [PUBMED Abstract]
- Röhrich M, Koelsche C, Schrimpf D, et al.: Methylation-based classification of benign and malignant peripheral nerve sheath tumors. Acta Neuropathol 131 (6): 877-87, 2016. [PUBMED Abstract]
- Kaplan HG, Rostad S, Ross JS, et al.: Genomic Profiling in Patients With Malignant Peripheral Nerve Sheath Tumors Reveals Multiple Pathways With Targetable Mutations. J Natl Compr Canc Netw 16 (8): 967-974, 2018. [PUBMED Abstract]
- Hagel C, Zils U, Peiper M, et al.: Histopathology and clinical outcome of NF1-associated vs. sporadic malignant peripheral nerve sheath tumors. J Neurooncol 82 (2): 187-92, 2007. [PUBMED Abstract]
- Zou C, Smith KD, Liu J, et al.: Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 249 (6): 1014-22, 2009. [PUBMED Abstract]
- Okada K, Hasegawa T, Tajino T, et al.: Clinical relevance of pathological grades of malignant peripheral nerve sheath tumor: a multi-institution TMTS study of 56 cases in Northern Japan. Ann Surg Oncol 14 (2): 597-604, 2007. [PUBMED Abstract]
- Amirian ES, Goodman JC, New P, et al.: Pediatric and adult malignant peripheral nerve sheath tumors: an analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol 116 (3): 609-16, 2014. [PUBMED Abstract]
- Valentin T, Le Cesne A, Ray-Coquard I, et al.: Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO). Eur J Cancer 56: 77-84, 2016. [PUBMED Abstract]
- Høland M, Kolberg M, Danielsen SA, et al.: Inferior survival for patients with malignant peripheral nerve sheath tumors defined by aberrant TP53. Mod Pathol 31 (11): 1694-1707, 2018. [PUBMED Abstract]
- Krawczyk MA, Karpinsky G, Izycka-Swieszewska E, et al.: Immunohistochemical assessment of cyclin D1 and p53 is associated with survival in childhood malignant peripheral nerve sheath tumor. Cancer Biomark 24 (3): 351-361, 2019. [PUBMED Abstract]
- Akshintala S, Mallory NC, Lu Y, et al.: Outcome of Patients With Malignant Peripheral Nerve Sheath Tumors Enrolled on Sarcoma Alliance for Research Through Collaboration (SARC) Phase II Trials. Oncologist 28 (5): 453-459, 2023. [PUBMED Abstract]
- Martin E, Coert JH, Flucke UE, et al.: Neurofibromatosis-associated malignant peripheral nerve sheath tumors in children have a worse prognosis: A nationwide cohort study. Pediatr Blood Cancer 67 (4): e28138, 2020. [PUBMED Abstract]
- Meister MT, Scheer M, Hallmen E, et al.: Malignant peripheral nerve sheath tumors in children, adolescents, and young adults: Treatment results of five Cooperative Weichteilsarkom Studiengruppe (CWS) trials and one registry. J Surg Oncol 122 (7): 1337-1347, 2020. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Ferrari A, Bisogno G, Macaluso A, et al.: Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer 109 (7): 1406-12, 2007. [PUBMED Abstract]
- van Noesel MM, Orbach D, Brennan B, et al.: Outcome and prognostic factors in pediatric malignant peripheral nerve sheath tumors: An analysis of the European Pediatric Soft Tissue Sarcoma Group (EpSSG) NRSTS-2005 prospective study. Pediatr Blood Cancer 66 (10): e27833, 2019. [PUBMED Abstract]
- Higham CS, Steinberg SM, Dombi E, et al.: SARC006: Phase II Trial of Chemotherapy in Sporadic and Neurofibromatosis Type 1 Associated Chemotherapy-Naive Malignant Peripheral Nerve Sheath Tumors. Sarcoma 2017: 8685638, 2017. [PUBMED Abstract]
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Al-Ezzi E, Gounder M, Watson G, et al.: Selinexor, a First in Class, Nuclear Export Inhibitor for the Treatment of Advanced Malignant Peripheral Nerve Sheath Tumor. Oncologist 26 (4): e710-e714, 2021. [PUBMED Abstract]
- WHO Classification of Tumours Editorial Board: WHO Classification of Tumours. Volume 3: Soft Tissue and Bone Tumours. 5th ed., IARC Press, 2020.
- de Traux de Wardin H, Dermawan JK, Vanoli F, et al.: NF1-Driven Rhabdomyosarcoma Phenotypes: A Comparative Clinical and Molecular Study of NF1-Mutant Rhabdomyosarcoma and NF1-Associated Malignant Triton Tumor. JCO Precis Oncol 8: e2300597, 2024. [PUBMED Abstract]
- Schaefer IM, Fletcher CD, Hornick JL: Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol 29 (1): 4-13, 2016. [PUBMED Abstract]
- Hornick JL, Nielsen GP: Beyond "Triton": Malignant Peripheral Nerve Sheath Tumors With Complete Heterologous Rhabdomyoblastic Differentiation Mimicking Spindle Cell Rhabdomyosarcoma. Am J Surg Pathol 43 (10): 1323-1330, 2019. [PUBMED Abstract]
Treatment of Pericytic (Perivascular) Tumors
Pericytic (perivascular) tumors have several subtypes, including the following:
Myopericytoma
Infantile hemangiopericytoma, a subtype of myopericytoma, is a highly vascularized tumor of uncertain origin.
For children with hemangiopericytomas, those younger than 1 year seem to have a better prognosis than children older than 1 year.[1-3]
Histology
Histologically, hemangiopericytomas are composed of packed round or fusiform cells that are arranged around a complex vasculature, forming many branch-like structures. Hyalinization is often present. Infantile hemangiopericytomas have similar histology but many are multilobular with vasculature outside the tumor mass.[4]
Treatment and outcome of infantile hemangiopericytomas
Treatment options for infantile hemangiopericytomas include the following:
- Chemotherapy.
Evidence (chemotherapy):
- In a series of 17 children, the differences in metastatic potential and response to treatment were clearly demonstrated for adult and infantile hemangiopericytomas. Eleven children were older than 1 year. Several of these patients had disease in the lymph nodes or lungs.[5]
- Three patients with stage I disease survived, although one patient had recurrence in the lungs.
- Eight patients had stage II or stage III disease. Two of these patients survived and six patients had disease progression and died.
- Six patients had infantile hemangiopericytoma, five of which were greater than stage I. All six patients survived, and three patients had good responses to vincristine, actinomycin, and cyclophosphamide.
Several studies have reported on soft tissue sarcomas in children that were more akin to infantile myofibromatosis or hemangiopericytoma.[6,7] Rather than the ETV6::NTRK3 fusion protein seen in congenital infantile fibrosarcoma, a LMNA::NTRK1 fusion protein was identified.[8] One patient carrying this fusion responded to crizotinib. For more information about infantile myofibromatosis, see the Infantile Myofibromatosis section.
Infantile Myofibromatosis
Infantile myofibromatosis is a fibrous tumor of infancy and childhood that most commonly presents in the first 2 years of life.[9]
The lesion can present as a single subcutaneous nodule (myofibroma) most commonly involving the head and neck region, or lesions can affect multiple skin areas, muscle, and bone (myofibromatosis).[10-13]
Genomic alterations and genetic testing
Somatic gain-of-function PDGFRB variants have been identified in sporadic cases of infantile myofibromatosis, including activating single nucleotide variants and in-frame indels and duplications.[14,15] PDGFRB variants are observed in most cases with multicentric nodules, but are less common in cases with solitary myofibroma.[15,16] Some PDGFRB variants that cause infantile myofibromatosis are sensitive to tyrosine kinase inhibitors like imatinib.[15,16]
An inherited autosomal dominant form of infantile myofibromatosis has been described. It is associated with germline pathogenic variants of the PDGFRB gene, with the R561C variant being most commonly observed.[17-19] The R561C variant is a relatively weak activator of PDGFRB, which may explain the presence of additional PDGFRB variants with stronger activity in some familial infantile myofibromatosis cases.[16,17]
The European Society for Paediatric Oncology Host Genome Working Group developed counseling and germline testing guidelines for these groups of children. This group recommends germline analysis for children with infantile myofibromatosis who have at least one of the following criteria:[20]
- Multicentric disease.
- First- or second-degree relatives with infantile myofibromatosis or soft tissue nodules during childhood.
- A known PDGFRB germline pathogenic variant in the family.
- Suspected germline mosaic PDGFRB pathogenic variants.
Treatment and outcome of infantile myofibromatosis
Patients with these tumors usually have an excellent prognosis and the tumors can regress spontaneously. However, about one-third of cases with multicentric involvement will also have visceral involvement, and the prognosis for these patients is poor.[12,13,21]
Treatment options for infantile myofibromatosis include the following:
- Observation.
- Chemotherapy.
- Tyrosine kinase inhibitors effective against PDGFRB.
Ninety-five patients were prospectively enrolled in five Cooperative Weichteilsarkom Studiengruppe (CWS) trials and one registry trial between 1981 and 2016.[22] Localized disease was diagnosed in 71 patients. Forty-two (59%) of these patients were infants younger than 12 months. The mainstay of treatment (applied to 55 children) was watch and wait after initial biopsy or resection. Systemic therapy was only recommended in cases of life-threatening progressive disease or in cases of compression of vital structures or organ dysfunction in the setting of progressive disease. Based on these criteria, chemotherapy was administered to 16 of 71 patients as an individual decision at the treating center: 8 patients received methotrexate/vinblastine, 5 patients received vincristine/dactinomycin/cyclophosphamide (VAC), and 3 patients received other therapies.
- Of the patients who received chemotherapy, nine could be assessed for response. Two patients experienced a complete remission (CR) or partial remission (PR) (objective response rate, 22%).
- Overall, 77 patients were alive in CR, and 10 patients were alive in PR. Three patients died of progressive disease.
- The 5-year event-free survival (EFS) rate was 73% for patients with localized disease and 51% for patients with multifocal disease.
- The 5-year overall survival (OS) rate was 95% for patients with localized or multifocal disease.
The use of combination chemotherapy with vincristine/dactinomycin and vinblastine/methotrexate have proven effective in cases of multicentric disease with visceral involvement and in cases in which the disease has progressed and has threatened the life of the patient (e.g., upper airway obstruction).[12,13,23]
Case reports have described prompt tumor regression in patients with infantile myofibromatosis that have PDGFRB variants when treated with tyrosine kinase inhibitors like imatinib and sunitinib, which inhibit the PDGFRB gain-of-function variant in the tumor.[24-27]
References
- Rodriguez-Galindo C, Ramsey K, Jenkins JJ, et al.: Hemangiopericytoma in children and infants. Cancer 88 (1): 198-204, 2000. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Hemangiopericytoma in pediatric ages: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 92 (10): 2692-8, 2001. [PUBMED Abstract]
- Bien E, Stachowicz-Stencel T, Godzinski J, et al.: Retrospective multi-institutional study on hemangiopericytoma in Polish children. Pediatr Int 51 (1): 19-24, 2009. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. Mosby, 2008.
- Fernandez-Pineda I, Parida L, Jenkins JJ, et al.: Childhood hemangiopericytoma: review of St Jude Children's Research Hospital. J Pediatr Hematol Oncol 33 (5): 356-9, 2011. [PUBMED Abstract]
- Haller F, Knopf J, Ackermann A, et al.: Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: a subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. J Pathol 238 (5): 700-10, 2016. [PUBMED Abstract]
- Wong V, Pavlick D, Brennan T, et al.: Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J Natl Cancer Inst 108 (1): , 2016. [PUBMED Abstract]
- Doebele RC, Davis LE, Vaishnavi A, et al.: An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101. Cancer Discov 5 (10): 1049-57, 2015. [PUBMED Abstract]
- Wiswell TE, Davis J, Cunningham BE, et al.: Infantile myofibromatosis: the most common fibrous tumor of infancy. J Pediatr Surg 23 (4): 315-8, 1988. [PUBMED Abstract]
- Chung EB, Enzinger FM: Infantile myofibromatosis. Cancer 48 (8): 1807-18, 1981. [PUBMED Abstract]
- Modi N: Congenital generalised fibromatosis. Arch Dis Child 57 (11): 881-2, 1982. [PUBMED Abstract]
- Levine E, Fréneaux P, Schleiermacher G, et al.: Risk-adapted therapy for infantile myofibromatosis in children. Pediatr Blood Cancer 59 (1): 115-20, 2012. [PUBMED Abstract]
- Larralde M, Hoffner MV, Boggio P, et al.: Infantile myofibromatosis: report of nine patients. Pediatr Dermatol 27 (1): 29-33, 2010 Jan-Feb. [PUBMED Abstract]
- Agaimy A, Bieg M, Michal M, et al.: Recurrent Somatic PDGFRB Mutations in Sporadic Infantile/Solitary Adult Myofibromas But Not in Angioleiomyomas and Myopericytomas. Am J Surg Pathol 41 (2): 195-203, 2017. [PUBMED Abstract]
- Arts FA, Sciot R, Brichard B, et al.: PDGFRB gain-of-function mutations in sporadic infantile myofibromatosis. Hum Mol Genet 26 (10): 1801-1810, 2017. [PUBMED Abstract]
- Dachy G, de Krijger RR, Fraitag S, et al.: Association of PDGFRB Mutations With Pediatric Myofibroma and Myofibromatosis. JAMA Dermatol 155 (8): 946-950, 2019. [PUBMED Abstract]
- Cheung YH, Gayden T, Campeau PM, et al.: A recurrent PDGFRB mutation causes familial infantile myofibromatosis. Am J Hum Genet 92 (6): 996-1000, 2013. [PUBMED Abstract]
- Martignetti JA, Tian L, Li D, et al.: Mutations in PDGFRB cause autosomal-dominant infantile myofibromatosis. Am J Hum Genet 92 (6): 1001-7, 2013. [PUBMED Abstract]
- Murray N, Hanna B, Graf N, et al.: The spectrum of infantile myofibromatosis includes both non-penetrance and adult recurrence. Eur J Med Genet 60 (7): 353-358, 2017. [PUBMED Abstract]
- Hettmer S, Dachy G, Seitz G, et al.: Genetic testing and surveillance in infantile myofibromatosis: a report from the SIOPE Host Genome Working Group. Fam Cancer 20 (4): 327-336, 2021. [PUBMED Abstract]
- Gopal M, Chahal G, Al-Rifai Z, et al.: Infantile myofibromatosis. Pediatr Surg Int 24 (3): 287-91, 2008. [PUBMED Abstract]
- Sparber-Sauer M, Vokuhl C, Seitz G, et al.: Infantile myofibromatosis: Excellent prognosis but also rare fatal progressive disease. Treatment results of five Cooperative Weichteilsarkom Studiengruppe (CWS) trials and one registry. Pediatr Blood Cancer 69 (3): e29403, 2022. [PUBMED Abstract]
- Weaver MS, Navid F, Huppmann A, et al.: Vincristine and Dactinomycin in Infantile Myofibromatosis With a Review of Treatment Options. J Pediatr Hematol Oncol 37 (3): 237-41, 2015. [PUBMED Abstract]
- Weller JM, Keil VC, Gielen GH, et al.: PDGRFB mutation-associated myofibromatosis: Response to targeted therapy with imatinib. Am J Med Genet A 179 (9): 1895-1897, 2019. [PUBMED Abstract]
- Wenger TL, Bly RA, Wu N, et al.: Activating variants in PDGFRB result in a spectrum of disorders responsive to imatinib monotherapy. Am J Med Genet A 182 (7): 1576-1591, 2020. [PUBMED Abstract]
- Mudry P, Slaby O, Neradil J, et al.: Case report: rapid and durable response to PDGFR targeted therapy in a child with refractory multiple infantile myofibromatosis and a heterozygous germline mutation of the PDGFRB gene. BMC Cancer 17 (1): 119, 2017. [PUBMED Abstract]
- Pond D, Arts FA, Mendelsohn NJ, et al.: A patient with germ-line gain-of-function PDGFRB p.N666H mutation and marked clinical response to imatinib. Genet Med 20 (1): 142-150, 2018. [PUBMED Abstract]
Treatment of Tumors of Uncertain Differentiation
Tumors of uncertain differentiation have many subtypes, including the following:
- Myxoma not otherwise specified (NOS) (benign).
- Synovial sarcoma NOS (poorly differentiated, spindle cell, and biphasic varieties).
- Epithelioid sarcoma.
- Alveolar soft part sarcoma.
- Clear cell sarcoma NOS.
- Extraskeletal myxoid chondrosarcoma.
- Desmoplastic small round cell tumor.
- Rhabdoid tumor NOS (extrarenal).
- Perivascular epithelioid tumor (PEComa), malignant.
- Undifferentiated sarcoma.
- Pleomorphic sarcoma, undifferentiated.
- Intracranial mesenchymal tumor.
- Round cell sarcoma, undifferentiated.
Myxoma NOS
Carney complex
Carney complex is an autosomal dominant syndrome caused by variants in the PRKAR1A gene, located on chromosome 17.[1] The syndrome is characterized by cardiac and cutaneous myxomas, pale brown to brown lentigines, blue nevi, primary pigmented nodular adrenocortical disease causing Cushing syndrome, and a variety of endocrine and nonendocrine tumors, including pituitary adenomas, thyroid tumors, and large cell calcifying Sertoli cell tumor of the testis.[1-3] There are published surveillance guidelines for patients with Carney complex that include cardiac, testicular, and thyroid ultrasonography.
For patients with the Carney complex, prognosis depends on the frequency of recurrences of cardiac and skin myxomas and other tumors.
For more information about the treatment of conditions related to Carney complex, see the following summaries:
Synovial Sarcoma NOS (Poorly Differentiated, Spindle Cell, and Biphasic Varieties)
Synovial sarcoma accounts for 9% of soft tissue sarcomas in patients younger than 20 years (see Table 1).
Synovial sarcoma is one of the most common nonrhabdomyosarcomatous soft tissue sarcoma (NRSTS) in children and adolescents. In a review of the Surveillance, Epidemiology, and End Results (SEER) Program database from 1973 to 2005, 1,268 patients with synovial sarcoma were identified. Approximately 17% of these patients were children and adolescents, and the median age at diagnosis was 34 years.[4] In addition, in the Children's Oncology Group (COG) ARST0332 (NCT00346164) and European paediatric Soft Tissue Sarcoma Study Group (EpSSG) 2005 protocols, synovial sarcoma was the single most common histological subtype.[5]
Clinical presentation
The most common primary tumor location is the extremities, followed by trunk and head and neck.[4] Rarely, a synovial sarcoma may arise in the heart or pericardium or appear with a pleuropulmonary presentation.[6-9]
The most common site of metastasis is the lung.[10,11] The risk of metastases is highly influenced by tumor size. Patients with tumors that are larger than 5 cm have an estimated 32-fold higher risk of developing metastases compared with patients who have tumors smaller than 5 cm.
The Cooperative Weichteilsarkom Studiengruppe (CWS) reported on 432 patients younger than 21 years diagnosed with synovial sarcoma between 1981 and 2018.[12] The study compared three age groups of patients: children (aged 0–12 years; n = 176), adolescents (aged 13–16 years; n = 178), and young adults (aged 17–21 years; n = 78).
- The proportion of invasive tumors was significantly higher in older patients (children, 33%; adolescents, 39%; and young adults, 54%; P = .009).
- The proportion of tumors larger than 10 cm (children, 13%; adolescents, 21%; and young adults, 31%; P = .006) and the presence of metastasis at first diagnosis were also higher in older patients (children, 6%; adolescents, 10%; and young adults, 21%; P = .001).
Histological features, diagnostic evaluation, and genomic alterations
Synovial sarcoma can be subclassified as the following types:
- Synovial sarcoma, spindle cell.
- Synovial sarcoma, biphasic.
- Synovial sarcoma, poorly differentiated.
The diagnosis of synovial sarcoma is made by immunohistochemical analysis, ultrastructural findings, and demonstration of the specific chromosomal translocation t(x;18)(p11.2;q11.2). This abnormality is specific for synovial sarcoma and is found in all morphological subtypes. Synovial sarcoma results in rearrangement of the SS18 gene on chromosome 18 with one of the subtypes (1, 2, or 4) of the SSX gene on chromosome X.[13,14] It is thought that the SS18::SSX fusion transcript promotes epigenetic silencing of key tumor suppressor genes.[15]
In one report, reduced SMARCB1 nuclear reactivity on immunohistochemical staining was seen in 49 cases of synovial sarcoma, suggesting that this pattern may help distinguish synovial sarcoma from other histologies.[16]
Prognostic factors
Favorable prognostic factors
Patients younger than 10 years have more favorable outcomes and clinical features than older patients.
Favorable clinical features include the following:[4,17-19]
- Extremity primary tumors.
- Smaller tumors.
- Localized disease.
- Response to chemotherapy was correlated with improved survival in one meta-analysis.
Unfavorable prognostic factors
The following studies have reported multiple factors associated with unfavorable outcomes:
- In a retrospective analysis of synovial sarcoma in children and adolescents who were treated in Germany and Italy, tumor size (>5 cm or ≤5 cm in greatest dimension) was an important predictor of event-free survival (EFS).[20] In this analysis, local invasiveness conferred an inferior probability of EFS, but surgical margins were not associated with clinical outcome.
- In a single-institution retrospective analysis of 111 patients with synovial sarcoma who were younger than 22 years at diagnosis, larger tumor size, greater depth in tissue, greater local invasiveness, and more proximal tumor location were associated with poorer overall survival (OS).[21][Level of evidence C1]
- A multicenter analysis included 219 children from various treating centers, including Germany, St. Jude Children's Research Hospital (SJCRH), Instituto Tumori, and MD Anderson Cancer Center. The study reported an estimated 5-year OS rate of 80% and an EFS rate of 72%.[19] In this analysis, an interaction between tumor size and invasiveness was observed. In multivariate analysis, patients with large or invasive tumors or with Intergroup Rhabdomyosarcoma Study (IRS) group III disease (localized, incompletely resected or with biopsy only) and group IV disease (metastases at diagnosis) had decreased OS. Treatment with radiation therapy was related to improved OS (hazard ratio [HR], 0.4; 95% confidence interval [CI], 0.2–0.7). In patients with IRS group III disease, objective response to chemotherapy (18 of 30 [60%]) correlated with improved survival.
- Expression and genomic index prognostic signatures have been studied in synovial sarcoma. Complex genomic profiles, with greater rearrangement of the genome, are more common in adults than in younger patients with synovial sarcoma and are associated with a higher risk of metastasis.[22]
- A review of 84 patients with localized synovial sarcoma who had information on fusion status (SS18::SSX) and histological grading found no difference in OS according to these criteria. However, for tumor size at diagnosis, the study showed that patients with tumors between 5 cm and 10 cm had a worse prognosis than those with smaller tumors (P = .02). Patients with tumors larger than 10 cm had an even worse OS (P = .0003).[23][Level of evidence C1]
- The German CWS group reviewed 27 evaluable patients younger than 21 years with pulmonary metastases among 296 patients with synovial sarcoma. All patients had metastasis to the lungs. The 5-year EFS rate was 26%, and the OS rate was 30%. The most important prognostic factor at presentation was that the metastases were limited to one lesion in one lung or one lesion in both lungs (a group they termed oligometastatic). Treatment elements associated with superior survival were adequate local therapy of the primary tumor and, if feasible, for the metastases. The use of whole-lung irradiation did not correlate with better outcomes.[24][Level of evidence C1]
- The EpSSG designed a genomic index for synovial sarcoma.[25][Level of evidence C2] Genomic index was defined as A2/C, where A is the total number of alterations (segmental gains and losses) and C is the number of involved chromosomes on array comparative genomic hybridization results. In a multivariate analysis of 61 pediatric, adolescent, and young adult patients (aged <25 years), high genomic index was an independent predictor of decreased EFS and OS.
- In adults, factors such as International Union Against Cancer/American Joint Committee on Cancer stage III and stage IVA, poor tumor necrosis, truncal location, elevated mitotic rate, older age, and higher histological grade have been associated with a worse prognosis.[26-28]
Treatment of synovial sarcoma
Treatment options for synovial sarcoma include the following:
Surgery alone
Evidence (surgery alone):
- The COG and the EpSSG reported a combined analysis of 60 patients younger than 21 years with localized synovial sarcoma prospectively assigned to surgery without adjuvant radiation therapy or chemotherapy.[31] Enrollment was limited to patients with initial complete resection with histologically free margins, with a grade 2 tumor of any size or a grade 3 tumor 5 cm or smaller.
- The 3-year EFS rate was 90% (median follow-up, 5.2 years; range, 1.9–9.1 years).
- All eight events were local tumor recurrence; no metastatic recurrences were seen.
- All patients with recurrent disease were effectively treated with second-line therapy, resulting in an OS rate of 100%.
- Therefore, the authors concluded that a surgery-only approach was optimal for patients who achieved an R0 resection (complete resection with negative microscopic margins) and had tumors smaller than 5 cm, regardless of grade.
Surgery and chemotherapy, with or without radiation therapy
Synovial sarcoma appears to be more sensitive to chemotherapy than many other soft tissue sarcomas. Children with synovial sarcoma seem to have a better prognosis than adults with synovial sarcoma.[11,28,32-37]
The most commonly used chemotherapy regimens for the treatment of synovial sarcoma incorporate ifosfamide and doxorubicin.[19,35,38] Response rates to the ifosfamide and doxorubicin regimen are higher than in other NRSTS.[39]
Evidence (surgery and chemotherapy with or without radiation therapy):
- Several treatment centers advocate chemotherapy after resection and radiation therapy for children and young adults with synovial sarcoma.[19,20,40-42]
- The International Society of Pediatric Oncology-Malignant Mesenchymal Tumors studies showed that select patients (young age, <5 cm resected tumors) with nonmetastatic synovial sarcoma treated with chemotherapy can have excellent outcomes in the absence of radiation therapy. However, it is still unclear whether that approach obviates an advantage of radiation for local or regional control.[41]
- A German trial suggested a benefit for postoperative chemotherapy in children with synovial sarcoma.[42]
- A meta-analysis also suggested that chemotherapy may provide benefit.[19]
- The COG reported an analysis of the subset of patients with synovial sarcoma treated on the ARST0332 (NCT00346164) trial. This was a prospective treatment assignment trial for patients younger than 30 years with NRSTS.[43] They analyzed the outcomes of 138 eligible patients.
- Overall, R0 resection or R1 resection (microscopically positive margins) of the primary tumor was achieved in 129 patients (93.5%): 69 patients (53.5%) at study entry and 60 patients (46.5%) after neoadjuvant chemotherapy. Of these, 104 patients (80.6%) had an R0 resection: 55 patients (53%) at study entry and 49 patients (47%) after neoadjuvant chemotherapy.
- In the 60 patients who received neoadjuvant chemotherapy, response was evaluable in 55 patients. Two patients (3.6%) had complete responses, 9 (16.4%) had partial responses, 41 (74.6%) had stable disease, and 3 (5.5%) had progressive disease. The tissue from 57 tumors was centrally reviewed after definitive resection. Forty-one tumors (72%) had less than 90% necrosis, and 16 tumors (28%) had 90% necrosis or more.
- The study prospectively defined three risk groups:
- Low risk (about 50% of population): Patients with nonmetastatic, grossly resected tumors, except patients who had tumors that were both high grade and >5 cm in maximal diameter.
- Intermediate risk (about 35% of population): Patients with nonmetastatic tumors that were both high grade and >5 cm in maximal diameter and patients with nonmetastatic, nonresectable tumors regardless of grade and size.
- High risk (about 15% of population): Patients with metastatic tumors, including those with metastases restricted to regional lymph nodes.
- For the 46 patients in the low-risk group, the 5-year EFS rate was 81.9% (95% CI, 69%–94.8%), and the OS rate was 97.7% (95% CI, 92.7%–100%).
- For the 23 patients in the intermediate-risk group (treatment arm C), the 5-year EFS rate was 64% (95% CI, 42.4%–85.8%), and the OS rate was 89.5% (95% CI, 75.3%–100%).
- For the 49 patients in the intermediate-risk group (treatment arm D), the 5-year EFS rate was 71.2% (95% CI, 56.5%–85.9%), and the OS rate was 86.5% (95% CI, 75.6%–97.3%).
- For the 21 patients in the high-risk group, the 5-year EFS rate was 7.6% (95% CI, 0%–22%), and the OS rate was 12.5% (95% CI, 0%–28.7%).
- The EpSSG performed a prospective study of patients younger than 21 years with synovial sarcoma (CCLG-EPSSG-NRSTS-2005 [NCT00334854]).[44][Level of evidence C1] Patients were stratified into the following three risk groups and nonrandomly assigned to treatment by risk group:
- Low-risk patients had IRS group I tumors less than 5 cm in size and nonaxial primary tumors.
- Intermediate-risk patients had no axial primary tumors and IRS group I tumors greater than 5 cm or IRS group II tumors.
- High-risk patients included all patients with axial primary sites (head and neck, lung and pleura, trunk, retroperitoneal), IRS group III tumors, or N1 tumors.
Outcomes for patients treated on the CCLG-EPSSG-NRSTS-2005 trial are described in Table 12.
Table 12. Event-Free Survival (EFS) and Overall Survival (OS) in Patients With Low-, Intermediate-, and High-Risk Synovial Sarcoma Treated on the CCLG-EPSSG-NRSTS-2005 Trial Risk Group Treatment 3-Year EFS Rate (%) 3-Year OS Rate (%) IRS = Intergroup Rhabdomyosarcoma Study; RT = radiation therapy. aChemotherapy was ifosfamide/doxorubicin, with doxorubicin omitted during radiation therapy. b59.4 Gy in cases without the option of secondary resection; 50.4 Gy as preoperative radiation therapy; 50.4, 54, and 59.4 Gy as postoperative radiation therapy, in the case of R0, R1, and R2 resections, respectively (no additional radiation therapy in the case of secondary complete resections with free margins, in children younger than 6 years). Low Surgery alone 92 100 Intermediate Surgery, 3–6 cycles chemotherapya, ± RTb 91 100 High (IRS group III) 3 cycles of chemotherapya, surgery, 3 additional cycles of chemotherapy, ± RTb 77 94 High (axial primary sites) Surgery, 6 cycles of chemotherapya, RTb 78 100 - The CWS reported results from a prospective trial for the treatment of patients with synovial sarcoma. Eligibility was restricted to patients with localized tumors with macroscopic residual disease after first surgery, before the initiation of systemic therapy (IRS III) and no clinically detectable metastatic disease. There were 145 patients in the study with a median age of 14.5 years (range, 0.2–33.2 years). The protocols recommended but did not require radiation therapy to be given before definitive tumor resection. Radiation therapy was administered to 115 patients (79%), and 23 patients did not receive radiation therapy (no information documented for 7 patients). Of the 115 irradiated patients, 57 were irradiated before tumor excision and 52 after tumor excision.[45]
- In this nonrandomized comparison, the sequencing of radiation therapy before definitive resection was associated with a statistically significant improvement in local recurrence-free survival rates, compared with definitive surgery before radiation therapy.
- Omission of radiation therapy was associated with an inferior outcome.
- Outcomes for patients are described in Table 13.
Table 13. Effects of Radiation Therapy Timing on Outcomes of Patients With Synovial Sarcoma Radiation Therapy Patients (No.) 5-Year EFS Rate 5-Year OS Rate 5-Year Local Recurrence-Free Survival Rate EFS = event-free survival; OS = overall survival. No radiation therapy 23 44% 57% 76% Radiation therapy before surgery 57 70% 83% 98% Radiation therapy after surgery 52 73% 82% 86%
Recurrent synovial sarcoma NOS
For patients with recurrent synovial sarcoma, the survival rate after relapse is poor (30%–40% at 5 years). Factors associated with outcome after relapse include duration of first remission (> or ≤ 18 months) and lack of a second remission.[46,47]
In a German experience, surgical resection of metastatic disease was the most common way to achieve a second complete remission.[47] Maintenance chemotherapy with oral trofosfamide, idarubicin, and etoposide or oral cyclophosphamide and intravenous vinblastine was administered on an individual basis.
A consortium of six European referral centers reported a retrospective review of patients younger than 21 years with recurrent synovial sarcoma. Among 41 patients, the first relapses occurred within 3 to 132 months (median, 18 months) after first diagnoses. The relapses were local in 34% of patients, metastatic in 54%, and both in 12%. Treatments at first relapse included surgery in 56% of patients, radiation therapy in 34%, and systemic therapy in 88%. In all, 36 patients received second-line medical treatment, which included chemotherapy in 32 patients (with 10 different regimens) and targeted therapy in 4 patients. No patient was included in early-phase clinical trials as second-line therapy. The overall response rate was 42%. The median EFS was 12 months, and the postrelapse 5-year EFS rate was 15.8%. The median OS was 30 months, and the postrelapse 5-year OS rate was 22.2%. In a Cox multivariable regression analysis, OS was significantly associated with time and type of relapse.[48]
Radiation therapy (stereotactic body radiation therapy) can be used to target select pulmonary metastases. This is usually considered after a minimum of one resection to confirm metastatic disease. Radiation therapy is particularly appropriate for patients with lesions that threaten air exchange because of their location adjacent to bronchi or cause pain by invading the chest wall.[49]
Between 70% to 80% of synovial sarcomas express NY-ESO-1, an immunogenic cancer testis antigen.[50] NY-ESO-1 can be targeted with adoptive transfer of T cells engineered to express NY-ESO-1c259, an affinity-enhanced T-cell receptor (TCR) targeting NY-ESO-1/LAGE1a.[51] The procedure to produce the genetically engineered T cells restricts their reactivity to a single HLA type. All clinical trials of this technology chose HLA-A*02 as the initial target and limited eligibility to patients whose tumors expressed NY-ESO-1 and who had HLA-A*02. In a multi-institutional trial, confirmed antitumor responses occurred in 50% of patients (6 of 12) and were characterized by tumor shrinkage over several months. Circulating NY-ESO-1c259 T cells were present postinfusion in all patients, and the cells persisted for at least 6 months in all responders.[52]
An open-label, international, phase II study enrolled patients with previously treated metastatic or unresectable synovial sarcoma and myxoid round cell liposarcoma.[53] Fifty-two patients with HLA-A*02 and tumors that expressed melanoma-associated antigen A4 (MAGE-A4) received a single intravenous dose of afamitresgene autoleucel (afami-cel) after lymphodepletion. Afami-cel is a MAGE-A4–directed, genetically modified, autologous T-cell immunotherapy. The overall response rate (the primary end point of the study) was 37% (19 of 52; 95% CI, 24%–51%), 39% (17 of 44; 95% CI, 24%–55%) for patients with synovial sarcoma, and 25% (2 of 8; 95% CI, 3%–65%) for patients with myxoid round cell liposarcoma. Cytokine release syndrome and cytopenias were the most frequent side effects. The authors concluded that afami-cel treatment resulted in durable responses in heavily pretreated eligible patients with synovial sarcoma.[53] These data led to FDA approval of afami-cel in adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA-A*02 positive, and whose tumors express the MAGE-A4 antigen.
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Epithelioid Sarcoma
Epithelioid sarcoma is a rare mesenchymal tumor of uncertain histogenesis that displays multilineage differentiation.[54]
Clinical presentation
Epithelioid sarcoma commonly presents as a slowly growing firm nodule based in the deep soft tissue. The proximal type predominantly affects adults and involves the axial skeleton and proximal sites. The tumor is highly aggressive and has a propensity for lymph node metastases.
Genomic alterations
Epithelioid sarcoma is characterized by inactivation of the SMARCB1 gene, which is present in both conventional and proximal types of epithelioid sarcoma.[55] This abnormality leads to increased dependence on EZH2 and tumor formation.[56]
Treatment of epithelioid sarcoma
Treatment options for epithelioid sarcoma include the following:
Surgery with or without chemotherapy and/or radiation therapy
Surgical removal of primary and recurrent tumor(s) is the most effective treatment.[57][Level of evidence C1] Because of the propensity of this disease to have occult metastasis to the lymph nodes, sentinel lymph node biopsy is recommended for epithelioid sarcoma of the extremities or buttocks in the absence of clinically (by imaging or physical examination) enlarged lymph nodes.[58]
Evidence (surgery with or without chemotherapy and/or radiation therapy):
- In a German CWS retrospective analysis of 67 children, adolescents, and young adults (median age, 14 years) with epithelioid sarcoma, 53 patients presented with localized disease and 14 patients presented with metastatic disease.[59][Level of evidence C1] Fifty-eight of 67 patients were treated with primary resections. Resections were microscopically complete in 35 patients, microscopically incomplete in 12 patients, and macroscopically incomplete in 20 patients. Forty-nine patients received chemotherapy, and 33 patients received radiation therapy.
- Complete remission was achieved in 45 of 53 patients (85%) with localized disease.
- Twenty-seven patients with localized disease had local (n = 16), metastatic (n = 6), or combined (n = 4) relapses after a median time of 0.9 years (range, 0.1–2.3 years) after complete response of disease (45 of 63).
- Patients with localized disease had a 5-year EFS rate of 35% (95% CI, ±12%) and an OS rate of 48% (95% CI, ±14%).
- Patients with metastatic disease had a 5-year EFS rate of 7% (95% CI, ±14%) and an OS rate of 9% (95% CI, ±16%).
- Smaller tumor size, lower IRS group, less tumor invasiveness, negative nodal status, and microscopically complete resection correlated with a favorable prognosis in patients with localized disease.
- A retrospective analysis reviewed COG and EpSSG prospective clinical trials that enrolled patients younger than 30 years with epithelioid sarcoma.[60][Level of evidence B4] The analysis identified 63 patients who were treated between July 2005 and November 2015. Patients were stratified into three risk groups using a combination of clinical features and treatment received. Low-risk patients (n = 34) underwent surgery with or without radiation therapy and included predominantly patients with nonmetastatic widely or marginally resected tumors 5 cm or smaller. The intermediate-risk group included patients (n = 16) with nonmetastatic, high-grade, and larger than 5 cm tumors or unresectable tumors. Patients with nodal or distant metastatic disease were at high risk (n = 13) , regardless of tumor grade or size.
- Partial responses were observed in 11 of 22 patients (50%) who received neoadjuvant therapy.
- Events were local recurrence (n = 10) and distant recurrence (n = 15).
- The estimated 5-year OS rates were 86.4% for low-risk patients, 63.5% for intermediate-risk patients, and 0% for high-risk patients.
- Locoregional nodal involvement, invasive tumor, high grade, and lesser extent of resection predicted poorer EFS in patients without metastases.
- A review of 30 pediatric patients with epithelioid sarcoma (median age at presentation, 12 years) reported the following results:[61]
- Responses to chemotherapy were reported in 40% of patients using sarcoma-based treatment regimens.
- Sixty percent of patients were alive at 5 years after initial diagnosis.
- A single-institution retrospective review of 20 patients, which included children and adults (median age, 27.3 years), reported the following:[57]
- There was no difference in the probability of recurrence between patients who received chemotherapy and those who did not receive chemotherapy.
- The authors suggested that radiation therapy may be useful.
Targeted therapy
Evidence (tazemetostat):
- In a phase II trial of 62 adult patients with epithelioid sarcoma and documented loss of INI1 by immunohistochemistry or biallelic SMARCB1 (the gene that encodes INI1) alterations, tazemetostat showed clinical activity.[62]
- There were 9 of 62 confirmed partial responses, with an objective response rate of 15% and a disease control rate of 26%.
In January 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to tazemetostat for adult and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma who were not eligible for complete resection.
Treatment options under clinical evaluation for epithelioid sarcoma
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- PEPN2121 (NCT05286801) (Tiragolumab and Atezolizumab for the Treatment of Relapsed or Refractory SMARCB1- or SMARCA4-Deficient Tumors): This study is evaluating the combination of a PD-L1 targeting antibody (atezolizumab) with a TIGIT targeting antibody (tiragolumab) for patients with SMARCB1- or SMARCA4-deficient tumors. Patients with epithelioid sarcoma may be eligible for this study.
Alveolar Soft Part Sarcoma
Alveolar soft part sarcomas account for 1.4% of soft tissue sarcomas in patients younger than 20 years (see Table 1).
Clinical presentation
The median age at presentation is 25 years for patients with alveolar soft part sarcoma. This tumor most commonly arises in the extremities but can occur in the oral and maxillofacial region.[63-65] Alveolar soft part sarcoma in children can present with evidence of metastatic disease.[66] Delayed metastases to the brain and lung are uncommon.[63]
In a series of 61 patients with alveolar soft part sarcoma who were treated in four consecutive CWS trials and the Soft Tissue Sarcoma Registry (SoTiSaR), 46 patients presented with localized disease and 15 patients had evidence of metastasis at diagnosis.[67]
Sixty-nine patients younger than 30 years with alveolar soft part sarcoma were treated between 1980 and 2014 at four major institutions. The median age at diagnosis was 17 years, and 64% of patients were female. The most common site of disease was the lower extremity, and 26 patients had an ASPSCR1::TFE3 gene translocation.[68]
Genomic alterations
This tumor of uncertain histogenesis is characterized by a consistent chromosomal translocation t(X;17)(p11.2;q25) that fuses the ASPSCR1 gene with the TFE3 gene.[69,70]
Prognosis
Alveolar soft part sarcoma in children may have an indolent course.[66] Patients with alveolar soft part sarcoma may relapse several years after a prolonged period of apparent remission.[67,71]
- In a series of 19 treated patients with alveolar soft part sarcoma, one study reported a 5-year OS rate of 80%. The OS rate was 91% for patients with localized disease, 100% for patients with tumors 5 cm or smaller, and 31% for patients with tumors larger than 5 cm.[72]
- In another series of 33 patients, the OS rate was 68% at 5 years from diagnosis and 53% at 10 years from diagnosis. Survival was better for patients with smaller tumors (≤5 cm) and completely resected tumors.[73][Level of evidence C1]
- A retrospective review of children and young adults younger than 30 years (median age, 17 years; range, 1.5–30 years) from four institutions identified 69 patients treated primarily with surgery between 1980 and 2014.[68][Level of evidence C1] The ASPSCR1::TFE3 translocation was present in all 26 patients tested. There were 19 patients with IRS group I tumors (28%), 7 patients with IRS group II tumors (10%), 5 patients with IRS group III tumors (7%), and 38 patients with IRS group IV tumors (55%). The 5-year EFS rate was 80%, and the OS rate was 87% for the 31 patients with localized tumors (IRS postsurgical groups I, II, and III). The 5-year EFS rate was 7%, and the OS rate was 61% for the 38 patients with metastatic tumors (IRS group IV).
- In a series of patients treated on consecutive studies from Germany, 15 of 61 patients (25%) presented with metastases, often miliary in nature. Despite lack of response to chemotherapy, the 5-year OS rate was 61%, with an EFS rate of 20%.[67]
Treatment of alveolar soft part sarcoma
Treatment options for alveolar soft part sarcoma include the following:
- Surgery with or without radiation therapy and chemotherapy.[29,30]
- Targeted therapy (tyrosine kinase inhibitors and checkpoint inhibitors).[74]
Surgery with or without radiation therapy and chemotherapy
The standard treatment approach is complete resection of the primary lesion.[72] If complete excision is not feasible, radiation therapy is administered.
Evidence (surgery with or without chemotherapy):
- A study from China reported on 18 patients with alveolar soft part sarcoma of the oral and maxillofacial region. Fifteen patients were younger than 30 years. Surgical removal with negative margins was the primary treatment.[65][Level of evidence C2]
- All patients survived, and only one patient had metastatic disease recurrence.
- In a series of patients treated on consecutive studies from Germany, the following was reported:[67]
- Progression-free survival (PFS) for patients without metastases on presentation appeared to improve with complete resection of the primary tumor.
- The 5-year EFS rate was 100% for patients with completely resected tumors, compared with 50% for patients with microscopic or gross residual disease.
- In a series of 51 pediatric patients aged 0 to 21 years with alveolar soft part sarcoma, the following was reported:[63][Level of evidence C1]
- The OS rate was 78% at 10 years, and the EFS rate was about 63%.
- Patients with localized disease (n = 37) had a 10-year OS rate of 87%.
- The 14 patients with metastases at diagnosis had a 10-year OS rate of 44%, partly resulting from the surgical removal of the primary tumor and lung metastases in some patients.
- Only 3 of 18 patients (17%) with measurable disease had a response to conventional antisarcoma chemotherapy, but two of four patients treated with sunitinib had a partial response.
Targeted therapy
Studies of targeted therapy (tyrosine kinase inhibitors and checkpoint inhibitors) have been done.
Sunitinib
Evidence (sunitinib):
- A small retrospective study of nine adult patients with metastatic alveolar soft part sarcoma treated with sunitinib reported partial responses in five patients and stable disease in two patients.[75][Level of evidence C3]
- In another study, 15 adult patients with alveolar soft part sarcoma were treated with sunitinib. Five patients were treated with sunitinib for longer than 2 years.[76][Level of evidence C1]
- Six patients experienced partial responses.
- The median PFS was 19 months, and the median OS was 56 months.
- The 5-year OS rate was 49%.
Cediranib
Cediranib is an inhibitor of all three known vascular epidermal growth factor receptors.
Evidence (cediranib):
- In a pediatric phase II trial of cediranib, using 70% of the adult maximum tolerated dose in patients younger than 16 years, the following was reported:[77][Level of evidence B4]
- Five of seven patients had stable disease for 14 months or longer.
- An international group performed a double-blind, placebo-controlled, randomized, phase II trial of cediranib in adolescent and adult patients with alveolar soft part sarcoma.[78][Level of evidence A1]
- Median percentage change in sum of target marker lesion diameters for the evaluable population was -8.3% (interquartile range [IQR], -26.5 to 5.9) for patients who received cediranib therapy, compared with 13.4% (IQR, 1.1–21.3) for patients who received the placebo (one-sided P = .0010).
- The authors concluded that cediranib is an active agent in patients with alveolar soft part sarcoma.
- In a phase II trial of cediranib, 15 of 43 adult patients (35%) with metastatic alveolar soft part sarcoma had partial responses.[79][Level of evidence C3]
Pazopanib
Evidence (pazopanib):
- In an open-label trial that evaluated the efficacy of pazopanib in six adult patients, one patient achieved a partial response and five patients had stable disease.[80]
- Another trial included 30 adult patients who were treated with pazopanib.[81]
- One patient experienced a complete response, seven patients experienced partial responses, and 17 patients had stable disease.
- The median PFS was 13.6 months.
Axitinib and pembrolizumab
Axitinib is a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Pembrolizumab is an anti–PD-1 immune checkpoint inhibitor.
Evidence (axitinib and pembrolizumab):
- In one trial, adult patients with advanced sarcomas were treated with a combination of axitinib and pembrolizumab.[74]
- For the 12 patients with alveolar soft part sarcoma, the 3-month PFS rate was 73%.
- Six of eleven patients with evaluable disease had partial responses to axitinib.
Atezolizumab
Atezolizumab is a monoclonal antibody directed against PD-1 and PD-L1.
Evidence (atezolizumab):
- In a phase II trial, 52 patients older than 2 years with advanced alveolar soft part sarcoma were treated with atezolizumab.[82]
- Nineteen patients experienced a response, 18 had partial responses and 1 had a complete response.
- Based on these data, the FDA approved the use of atezolizumab in children older than 2 years with unresectable or metastatic alveolar soft part sarcoma.
Other therapies
There have been sporadic reports of objective responses to treatment with interferon-alpha and bevacizumab.[63,83,84]
Because these tumors are rare, all children with alveolar soft part sarcoma should be considered for enrollment in prospective clinical trials. Information about ongoing clinical trials is available from the NCI website.
Treatment options under clinical evaluation for alveolar soft part sarcoma
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Clear Cell Sarcoma NOS
Clear cell sarcoma (formerly called malignant melanoma of soft parts) is a rare soft tissue sarcoma that typically involves the deep soft tissues of the extremities. It is also called clear cell sarcoma of tendons and aponeuroses. The tumor often affects adolescents and young adults.
Clinical presentation
The tumor most commonly affects the lower extremity, particularly the foot, heel, and ankle.[85,86] It has a high propensity for nodal dissemination, especially metastases to regional lymph nodes (12%–43%).[86,87]
The tumor typically has an indolent clinical course. Patients who have small, localized tumors with low mitotic rate and intermediate histological grade have the best outcomes.[88]
Genomic alterations
Clear cell sarcoma of soft tissue is characterized by EWSR1::ATF1 or EWSR1::CREB1 gene fusions.[89,90]
Treatment of clear cell sarcoma of soft tissue
Treatment options for clear cell sarcoma of soft tissue include the following:
Surgery with or without radiation therapy
Surgery with or without radiation therapy is the treatment of choice and offers the best chance for cure.
Evidence (surgery with or without radiation therapy):
- In a series of 28 pediatric patients reported by the Italian and German Soft Tissue Cooperative Studies, the median age at diagnosis was 14 years and the lower extremity was the most common primary site (50%).[91]; [92][Level of evidence C2]
- In this series, 12 of 13 patients with completely resected tumors were cured.
- For patients with more advanced disease, the outcome is poor and chemotherapy is rarely effective.
Targeted therapy
Evidence (targeted therapy):
- In a study by the European Organization for Research and Treatment of Cancer, 26 patients with clear cell sarcoma who had metastatic disease and documented EWSR1 rearrangements were treated with crizotinib.[93]
- One patient had a partial response, and 17 patients had stable disease.
Extraskeletal Myxoid Chondrosarcoma
Extraskeletal myxoid chondrosarcoma is relatively rare among soft tissue sarcomas, representing only 2.3% of all soft tissue sarcomas.[94] It has been reported in children and adolescents.[95]
The tumor has traditionally been considered to have low-grade malignant potential.[96] However, reports from large institutions showed that extraskeletal myxoid chondrosarcoma has significant malignant potential, especially if patients are monitored for a long time.[97,98] Patients tend to have slow protracted courses. Nodal involvement has been well described. Local recurrence (57%) and metastatic spread to lungs (26%) have been reported.[98]
Genomic alterations
Extraskeletal myxoid chondrosarcoma is a multinodular neoplasm. The rounded cells are arranged in cords and strands in a chondroitin sulfate myxoid background. Several cytogenetic abnormalities have been identified (see Table 2), with the most frequent being the EWSR1::NR4A3 gene fusion.[99]
Treatment of extraskeletal myxoid chondrosarcoma
Treatment options for extraskeletal myxoid chondrosarcoma include the following:
- Surgery.
- Radiation therapy.
Aggressive local control and resection of metastases led to OS rates of 87% at 5 years and 63% at 10 years. Tumors were relatively resistant to radiation therapy.[97] The therapeutic benefit of chemotherapy has not been established.
There may be potential genetic targets for small molecules, but these need to be studied as part of a clinical trial. In an adult study, six of ten patients who received sunitinib achieved partial responses.[100]
Extraskeletal Ewing Sarcoma
Almost one-fifth of patients with Ewing sarcoma will present with nonbone primary sites (extraosseous). Treatment for this tumor is the same as it is for patients with bone primary tumors.[101] For more information, see Ewing Sarcoma and Undifferentiated Small Round Cell Sarcomas of Bone and Soft Tissue Treatment.
Desmoplastic Small Round Cell Tumor
Desmoplastic small round cell tumor is a rare primitive sarcoma.
Clinical presentation
Desmoplastic small round cell tumor most frequently involves the peritoneum in the abdomen, pelvis, and/or peritoneum into the scrotal sac, but it may occur in the kidney or other solid organs.[102-106] Dozens to hundreds of intraperitoneal implants are often found. The tumor occurs predominantly in males (85%) and may spread to the lungs and elsewhere.[106,107]
Diagnostic evaluation
A large single-institution series of 65 patients compared computed tomography (CT) scans (n = 54) with positron emission tomography (PET)-CT scans (n = 11). PET-CT scans produced very few false-negative results and detected metastatic sites missed on conventional CT scans.[107]
Genomic alterations
Cytogenetic studies of these tumors have demonstrated the recurrent translocation t(11;22)(p13;q12), which has been characterized as a fusion of the WT1 and EWSR1 genes.[105,108] The EWSR1::WT1 fusion confirms the diagnosis of desmoplastic small round cell tumor. The average tumor variant burden is low for desmoplastic small round cell tumor (<1 variant per megabase), and recurring gene alterations other than the EWSR1::WT1 fusion are uncommon.[109] A small percentage of cases (approximately 3%) have activating variants in FGFR4, with amplification of FGFR4 observed at similar frequency.[109,110] Inactivating variants in TP53 and ARID1A are observed in a small percentage of desmoplastic small round cell tumor cases.[109,110]
Prognosis
The overall prognosis for patients with desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Greater than 90% tumor resection either at presentation or after preoperative chemotherapy may be a favorable prognostic factor for OS.[111,112]; [113][Level of evidence C1] Response to neoadjuvant chemotherapy and complete resection (near 100%) is associated with improved outcome.[106,114]
Treatment of desmoplastic small round cell tumor
There is no standard approach to the treatment of desmoplastic small round cell tumor.
Treatment options for desmoplastic small round cell tumor include the following:
- Multimodality therapy (chemotherapy, surgery, and radiation therapy).
- Surgery with hyperthermic intraperitoneal chemotherapy (HIPEC).
- Other treatment options.
Multimodality therapy
Complete surgical resections are rare and usually performed in highly specialized centers, but are critical for any improved survival. Successful treatment modalities include neoadjuvant Ewing-type chemotherapy, followed by complete surgical resection of the extensive intra-abdominal tumors, followed by total abdominal radiation therapy. With this multimodality approach, survival can be achieved in 30% to 40% of patients at 5 years.[102,103,111,115-118]
Surgery with HIPEC
HIPEC is a local treatment method that may control more of the microscopic intra-abdominal disease. The theory is that the heated chemotherapy that is instilled in the abdominal cavity after surgical resection (at the time of surgery) provides synergistic cytotoxicity to any microscopic cells remaining in the abdomen.[119]
The addition of HIPEC to complete surgical resection (cytoreductive surgery) is a new technique first applied to children in 2006 in a phase I clinical trial. Cytoreductive surgery and HIPEC for desmoplastic small round cell tumors is part of a multidisciplinary approach and is only being done in highly specialized centers. Surgeries can last more than 12 hours, and technical aspects of this unique tumor resection should be considered.[119]
Evidence (surgery with HIPEC):
- A single-institution phase II study showed HIPEC to be a potentially promising addition to complete surgical resection. Fourteen patients with desmoplastic small round cell tumor and five patients with other sarcomas were enrolled. These highly selected patients had tumor limited to the abdominal cavity. They demonstrated a partial response to neoadjuvant Ewing-type chemotherapy, had complete surgical resections and received HIPEC using cisplatin. They also received adjuvant total-abdominal radiation therapy followed by adjuvant chemotherapy.[119]
- With this standardized approach, patients with desmoplastic small round cell tumors had an OS rate of 80% at 30 months and 40% at 50 months.
- Patients with desmoplastic small round cell tumors without liver metastasis had no intra-abdominal recurrences, whereas 87% of patients with liver metastasis or portal disease had a recurrence.
- In a retrospective study from centers in France, patients were treated with cytoreductive surgery and HIPEC. Twenty-two patients were selected, and the median age at diagnosis was 14.8 years (range, 4.2–17.6 years). Seven patients had peritoneal mesotheliomas, seven patients had desmoplastic small round cells tumors, and eight patients had other histological tumor types. A complete macroscopic resection (CC-0, where CC is completeness of cytoreduction) was achieved in 16 cases (73%). Four of the seven patients with desmoplastic small round cell tumors had complete resections.[120][Level of evidence C1]
- Sixteen patients (72%) experienced relapses after a median time of 9.6 months (range, 1.4–86.4 months).
- Nine patients (41%) died of relapsed disease after a median time of 5.3 months (range, 0.1–36.1 months).
- Another study from France reviewed the use of cytoreductive surgery and HIPEC for the treatment of patients with desmoplastic small round cell tumors who had disease limited to the abdomen. In 107 patients with desmoplastic small round cell tumors, 48 had no extraperitoneal metastasis and underwent cytoreductive surgery. Of 48 patients (mean age, 22 years), 38 (79%) received preoperative and/or postoperative chemotherapy, and 23 (48%) received postoperative whole-abdominopelvic radiation therapy. Intraperitoneal chemotherapy was administered to 11 patients (23%), 2 of whom received early postoperative intraperitoneal chemotherapy (EPIC) and 9 of whom received HIPEC.[121]
- After a median follow-up of 30 months, the median OS of the entire cohort was 42 months.
- The 2-year OS rate was 72%, and the 5-year OS rate was 19%.
- The 2-year disease-free survival (DFS) rate was 30%, and the 5-year DFS rate was 12%.
- Whole-abdominopelvic radiation therapy was the only variable associated with longer peritoneal recurrence-free survival and DFS after cytoreductive surgery.
- Of 11 patients who received intraperitoneal chemotherapy (HIPEC or EPIC), six different chemotherapy regimens were used. The survival or outcome of this group is not reported in the manuscript.
- The influence of HIPEC/EPIC on OS and DFS was not statistically significant, but standardized regimens were not used in all patients, making results difficult to determine.
- A single-institutional retrospective study reported on nine patients (median age, 19 years) with desmoplastic small round cell tumor. Most patients had widespread disease, including four patients with extra-abdominal disease and five patients with liver involvement. These nine patients underwent ten cytoreductive and HIPEC treatments. Additionally, seven patients also received radiation therapy, and three patients underwent stem cell transplant.[122]
- The 3-year relapse-free survival rate was 13%, and the OS rate was 55%.
- Therapy was often associated with prolonged hospitalizations.
- Long-term parenteral nutrition was required in eight patients for a median of 261 days.
- Other long-term complications included gastroparesis (n = 1), small bowel obstruction (n = 3), and hemorrhagic cystitis (n = 2).
Other treatment options
The Center for International Blood and Marrow Transplant Research analyzed patients with desmoplastic small round cell tumor in their registry who received consolidation with high-dose chemotherapy and autologous stem cell reconstitution.[123] While this retrospective registry analysis suggested some benefit to this approach, other investigators have abandoned the approach because of excessive toxicity and lack of efficacy.[111]
A single-institution study reported that five of five patients with recurrent desmoplastic small round cell tumor had partial responses to treatment with the combination of vinorelbine, cyclophosphamide, and temsirolimus.[124]
Rhabdoid Tumor NOS (Extrarenal)
Malignant rhabdoid tumors were first described in children with renal tumors in 1981. These tumors were later found in a variety of extrarenal sites. These tumors are uncommon and highly malignant, especially in children younger than 2 years. For more information, see the Rhabdoid Tumors of the Kidney section in Wilms Tumor and Other Childhood Kidney Tumors Treatment.
Extrarenal (extracranial) rhabdoid tumors account for 2% of soft tissue sarcomas in patients younger than 20 years (see Table 1).
Genetic and genomic alterations
The first sizeable series of children with extrarenal extracranial malignant rhabdoid tumor of soft tissues came from 26 patients enrolled on the IRS I through III studies during a review of pathology material. Only five patients (19%) were alive without disease beyond 2 years.[125]
Investigation of children with atypical teratoid/rhabdoid tumors of the brain, as well as those with renal and extrarenal malignant rhabdoid tumors, found germline pathogenic variants and acquired variants of the SMARCB1 gene in all 29 tumors tested.[126] Rhabdoid tumors may be associated with germline pathogenic variants of the SMARCB1 gene and may be inherited from an apparently unaffected parent.[127] This observation was extended to 32 malignant rhabdoid tumors at all sites in patients whose mean age at diagnosis was 12 months.[128]
Genetic testing and surveillance
Germline analysis should be considered for individuals of all ages with rhabdoid tumors. Genetic counseling is also part of the treatment plan, given the low-but-actual risk of familial recurrence. In cases of variants, parental screening should be considered, although such screening carries a low probability of positivity. Prenatal diagnosis can be performed in situations where a specific SMARCB1 variant or deletion has been documented in the family.[127]
To date, there is little evidence regarding the effectiveness of surveillance for patients with rhabdoid tumor predisposition syndrome type 1 caused by loss-of-function germline SMARCB1 pathogenic variants. However, because of the aggressive nature of the tumors with significant lethality and young age of onset in SMARCB1 carriers with truncating variants, consensus recommendations have been developed. These recommendations were developed by a group of pediatric cancer genetic experts (including oncologists, radiologists, and geneticists). They have not been formally studied to confirm the benefit of monitoring patients with germline SMARCB1 pathogenic variants. Given the potential survival benefit of surgically resectable disease, it is postulated that early detection might improve OS.[129-131]
Surveillance for patients with germline SMARCB1 pathogenic variants includes the following:
- Brain magnetic resonance imaging (MRI) every 3 months from birth (or diagnosis) until age 5 years.
- Abdominal ultrasonography with a focus on the kidneys every 3 months.
For information about SMARCB1 and rhabdoid tumor predisposition syndrome type 1, see Rhabdoid Tumor Predisposition Syndrome Type 1.
Prognosis and clinical presentation
Young age and metastatic disease at presentation are associated with poor outcomes in children with extracranial rhabdoid tumors.
One study that used data from the National Cancer Database identified 202 patients (aged younger than 18 years) with non–central nervous system (CNS) malignant rhabdoid tumors. The primary site of the malignant rhabdoid tumor was soft tissue (46%), kidney (45%), and liver (9%).[132]
- The 1-year OS rate was 48.8%, and the 5-year OS rate was 35.9%.
- The multivariate analysis demonstrated that age younger than 1 year and presence of metastasis were negative prognostic indications (P = .058).
- In the cohort of surgical patients (n = 143), there was a trend for an association between the presence of residual disease and a clinically significant worse outcome (HR, 1.54; 95% CI, 0.88–2.69; P = .13).
A SEER study examined 229 patients with renal, CNS, and extrarenal malignant rhabdoid tumor. Patients aged 2 to 18 years, patients with a limited extent of tumor, and patients who received radiation therapy had favorable outcomes compared with other patients (P < .002 for each comparison). Site of the primary tumor was not prognostically significant. The OS rate was 33% at 5 years.[133]
A European registry for extracranial rhabdoid tumors identified 100 patients from 14 countries between 2009 and 2018.[134] Half of the patients were younger than 1 year at diagnosis. In 30 patients (30%), the tumor was located in the kidneys. Extracranial, extrarenal malignant rhabdoid tumor was found in 70% of patients (70 of 100), and the most common locations were in the cervical region, thoracic region, and liver. Nine patients demonstrated synchronous tumors. Distant metastases at diagnosis were present in 35% of patients (35 of 100). SMARCB1 germline pathogenic variants were detected in 21% of patients (17 of 81 evaluable). The 5-year OS rate was 45.8% (± 5.4%), and the EFS rate was 35.2% (± 5.1%). In an adjusted multivariate model, presence of a germline pathogenic variant, metastasis, and lack of a gross-total resection were the strongest significant negative predictors of outcome.
Treatment of extrarenal (extracranial) rhabdoid tumor
Treatment options for extrarenal (extracranial) rhabdoid tumor include the following:[135-137][Level of evidence C1]
- Surgical removal when possible.
- Chemotherapy as used for soft tissue sarcomas (but no single regimen is currently accepted as best).
- Radiation therapy.
Responses to alisertib have been documented in four patients with CNS atypical teratoid/rhabdoid tumors.[138] For more information about CNS atypical teratoid/rhabdoid tumors, see Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- PEPN2121 (NCT05286801) (Tiragolumab and Atezolizumab for the Treatment of Relapsed or Refractory SMARCB1- or SMARCA4-Deficient Tumors): This study is evaluating the combination of a PD-L1 targeting antibody (atezolizumab) with a TIGIT targeting antibody (tiragolumab) for patients with SMARCB1- or SMARCA4-deficient tumors. Patients with extrarenal (extracranial) rhabdoid tumors may be eligible for this study.
PEComa, Malignant
Clinical presentation
PEComas occur in various rare gastrointestinal, pulmonary, gynecological, and genitourinary sites. Soft tissue, visceral, and gynecological PEComas are more commonly seen in middle-aged female patients and are usually not associated with the tuberous sclerosis complex.[139] The disease course may be indolent.
Risk factors and molecular features
Benign PEComas are common in patients with tuberous sclerosis, an autosomal dominant syndrome that also predisposes to renal cell cancer and brain tumors. Tuberous sclerosis is caused by germline pathogenic inactivation of either TSC1 (9q34) or TSC2 (16p13.3), and the same tumor suppressor genes are inactivated somatically in sporadic PEComas.[140] Inactivation of either gene results in stimulation of the mTOR pathway, providing the basis for the treatment of nonsurgically curable tumors with similar genetic inactivation (lymphangioleiomyomatosis and angiomyolipoma) with mTOR inhibitors.[141,142] A small proportion of PEComas have TFE3 rearrangements with fusions involving various genes, including SFPQ and RAD51B.[143]
Prognosis
Most PEComas have a benign clinical course, but malignant behavior has been reported and can be predicted based on the size of the tumor, mitotic rate, and presence of necrosis.[144]
Treatment of PEComas
There are no standard treatment options. Treatment may include surgery or observation followed by surgery when the tumor is large.[145]
In tumors with evidence of mTORC1 activation and TSC1 or TSC2 loss, including lymphangioleiomyomatosis and angiomyolipoma,[141] clinical activity using mTOR inhibitors, such as sirolimus, has been well documented. In a small case series, three adult patients with PEComas responded to sirolimus.[146]
In a phase II trial, 34 patients with metastatic or locally advanced malignant PEComas were treated with sirolimus protein-bound particles for injectable suspension (albumin-bound) (nab-sirolimus). Of the 31 patients eligible for efficacy analysis, 12 (39%) had a response (1 complete response and 11 partial responses), 16 (52%) had stable disease, and 3 (10%) had progressive disease. Responses were rapid and durable. The median duration of response was not reached after a median follow-up of 2.5 years. Treatment was ongoing for 7 of 12 patients who responded to treatment (range, 5.6 months to longer than 47.2 months). Tumor variant profiling was completed for 25 specimens. Eight of nine patients with TSC2 variants responded to treatment, while only 2 of 16 patients without TSC2 variants responded. In addition, responses were noted in 10 of 17 patients with phospho-S6 (pS6) expression. No response was noted in eight patients without pS6 expression. The absence of pS6 expression reflects the lack of mTORC1 activation.[147][Level of evidence C1] In 2021, the FDA approved nab-sirolimus for adult patients with PEComas.
Undifferentiated Sarcoma
From 1972 to 2006, patients with undifferentiated soft tissue sarcoma were eligible for participation in rhabdomyosarcoma trials coordinated by the IRS group and the COG. The rationale was that patients with undifferentiated soft tissue sarcoma had sites of disease and outcomes that were similar to those in patients with alveolar rhabdomyosarcoma. Therapeutic trials for adults with soft tissue sarcoma include patients with undifferentiated soft tissue sarcoma and other histologies, which are treated similarly, using ifosfamide and doxorubicin, and sometimes with other chemotherapy agents, surgery, and radiation therapy.
In the COG ARST0332 (NCT00346164) trial, patients with high-grade undifferentiated sarcoma were treated with an ifosfamide- and doxorubicin-based regimen. Results for the patients with high-grade undifferentiated sarcoma were reported together with all high-grade soft tissue sarcomas in the trial. The estimated 5-year EFS rate was 64% and the OS rate was 77% for sarcomas classified as high grade by the Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLC).[5][Level of evidence C1]
In a report of 32 patients with undifferentiated soft tissue sarcomas who were enrolled on the ARST0332 (NCT00346164) trial, the median age at enrollment was 13.6 years, and two-thirds of the patients were male. The most common primary sites were the paraspinal region and extremities. Five patients presented with metastatic disease.[148]
- The 5-year EFS rate was 71%, and the OS rate was 83%.
- Of the nine children with low-risk disease (localized low-grade resected disease or localized high-grade disease <5 cm resected with negative margins) who were treated with surgery or radiation therapy only, the 5-year EFS rate was 65% and the OS rate was 100%, suggesting that patients with low-risk disease can be salvaged if the disease recurs.
- The remaining 23 patients had either intermediate-risk disease (resected high-grade tumor >5 cm, unresected high-grade tumor >5 cm) or high-risk disease (metastasis to lymph nodes or distant sites) and were treated with chemoradiation therapy and delayed surgery when feasible. The 5-year EFS rate was 73%, and the OS estimate was 77%.
- Copy number aberrations were common, most frequently involving loss of 1p (25%), gain of 1q (25%), gain of chromosome 8 (25%), and gain of chromosome 2 (16%). These alterations were more commonly seen in patients with intermediate-risk or high-risk tumors, and there was a strong association between loss of chromosome 1p or gain of chromosome 1q and inferior clinical outcomes. Co-occurrence of 1q gain and 1p loss was associated with a particularly poor clinical outcome (5-year EFS and OS rates of 20%). Next-generation sequencing identified oncogenic fusions in eight of ten tumor samples, which included BCOR and CIC rearrangements, as well as COL1A1::PDGFB, KIAA1549::BRAF, and SAMD5::SASH1 gene fusions.
Pleomorphic Sarcoma, Undifferentiated (Malignant Fibrous Histiocytoma)
At one time, malignant fibrous histiocytoma was the single most common histotype among adults with soft tissue sarcomas. Since it was first recognized in the early 1960s, malignant fibrous histiocytoma has been controversial, in terms of both its histogenesis and its validity as a clinico-pathological entity. The World Health Organization (WHO) classification no longer includes malignant fibrous histiocytoma as a distinct diagnostic category but rather as a subtype of an undifferentiated pleomorphic sarcoma.[149,150]
This entity accounts for 2% to 6% of all childhood soft tissue sarcomas.[151]
Clinical presentation
These tumors occur mainly in the second decade of life. In a series of ten patients, the median age was 10 years, and the tumor was most commonly located in the extremities. In this series, all tumors were localized, and five of nine patients (for whom follow-up was available) were alive and in first remission.[151]
In another series of 17 pediatric patients with malignant fibrous histiocytoma (now classified as undifferentiated pleomorphic sarcoma), the median age at diagnosis was 5 years and the extremities were involved in eight cases.[152] All patients with metastatic disease died, and two patients experienced a clinical response to a doxorubicin-based regimen.
For more information about the treatment of malignant fibrous histiocytoma of bone, see Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment.
Risk factors
These tumors can arise in previously irradiated sites or as a second malignancy in patients with retinoblastoma.[153]
Molecular features
An analysis of 70 patients who were diagnosed with malignant fibrous histiocytosis of no specific type, storiform or pleomorphic malignant fibrous histiocytoma, pleomorphic sarcoma, or undifferentiated pleomorphic sarcoma showed a highly complex karyotype with no specific recurrent aberrations.[154]
Undifferentiated sarcomas with 12q13–15 amplification, including MDM2 and CDK4, are best classified as dedifferentiated liposarcomas.[154] The relationship between this tumor and the family of undifferentiated/unclassified tumors with spindle cell morphology remains relatively undefined.
Treatment of newly diagnosed pleomorphic sarcoma
For information about the treatment of undifferentiated pleomorphic sarcoma of bone, see Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment.
Treatment of recurrent or refractory pleomorphic sarcoma
Treatment options for recurrent or refractory pleomorphic sarcoma include the following:
- Pembrolizumab.
The Sarcoma Alliance for Research through Collaboration conducted a phase II trial of the checkpoint inhibitor pembrolizumab in patients aged 18 years and older with recurrent soft tissue sarcoma.[155][Level of evidence C3]
- Seven of 40 patients (18%) with soft tissue sarcoma had an objective response.
- Four of ten patients (40%) with undifferentiated pleomorphic sarcoma, two of ten patients (20%) with liposarcoma, and one of ten patients (10%) with synovial sarcoma had objective responses.
- No patients with leiomyosarcoma (n = 10) had an objective response.
Intracranial Mesenchymal Tumor
Intracranial mesenchymal tumor, with the FET::CREB gene fusion, has previously been called angiomatoid fibrous histiocytoma (AFP) or intracranial myxoid mesenchymal tumor. The molecular findings suggest that these tumors are histological variants of intracranial mesenchymal tumor.[156] In one study, the tumors of 20 patients were separated into two epigenetic subgroups. Group A tumors clustered nearest to but independent of solitary fibrous tumor and occurred in adolescents and young adults. Group B tumors clustered nearest to but independent of clear cell sarcoma and occurred in children. Patients with group B tumors had an inferior survival compared with patients with group A tumors (4.5 vs. 49 months; P = .001).[157]
Round Cell Sarcoma, Undifferentiated
Undifferentiated small round cell sarcomas with BCOR genetic alterations
See the sections on Undifferentiated Small Round Cell Sarcomas With BCOR Genetic Alterations and Genomics of Ewing Sarcoma in Ewing Sarcoma and Undifferentiated Small Round Cell Sarcomas of Bone and Soft Tissue Treatment.
Undifferentiated small round cell sarcomas with CIC genetic alterations
See the sections on Undifferentiated Small Round Cell Sarcomas With CIC Genetic Alterations and Genomics of Ewing Sarcoma in Ewing Sarcoma and Undifferentiated Small Round Cell Sarcomas of Bone and Soft Tissue Treatment.
Undifferentiated small round cell sarcomas with EWSR1::non-ETS fusions
See the Undifferentiated Small Round Cell Sarcomas With EWSR1::non-ETS Fusions section in Ewing Sarcoma and Undifferentiated Small Round Cell Sarcomas of Bone and Soft Tissue Treatment.
References
- Wilkes D, Charitakis K, Basson CT: Inherited disposition to cardiac myxoma development. Nat Rev Cancer 6 (2): 157-65, 2006. [PUBMED Abstract]
- Carney JA, Young WF: Primary pigmented nodular adrenocortical disease and its associated conditions. Endocrinologist 2: 6-21, 1992.
- Ryan MW, Cunningham S, Xiao SY: Maxillary sinus melanoma as the presenting feature of Carney complex. Int J Pediatr Otorhinolaryngol 72 (3): 405-8, 2008. [PUBMED Abstract]
- Sultan I, Rodriguez-Galindo C, Saab R, et al.: Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer 115 (15): 3537-47, 2009. [PUBMED Abstract]
- Spunt SL, Million L, Chi YY, et al.: A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children's Oncology Group prospective study. Lancet Oncol 21 (1): 145-161, 2020. [PUBMED Abstract]
- Wang JG, Li NN: Primary cardiac synovial sarcoma. Ann Thorac Surg 95 (6): 2202-9, 2013. [PUBMED Abstract]
- Chirmade PC, Parikh S, Anand A, et al.: Primary pleuropulmonary synovial sarcoma with brain metastases in a paediatric patient: an unusual presentation. Adv Respir Med 85 (4): 206-210, 2017. [PUBMED Abstract]
- Frazier AA, Franks TJ, Pugatch RD, et al.: From the archives of the AFIP: Pleuropulmonary synovial sarcoma. Radiographics 26 (3): 923-40, 2006 May-Jun. [PUBMED Abstract]
- Essary LR, Vargas SO, Fletcher CD: Primary pleuropulmonary synovial sarcoma: reappraisal of a recently described anatomic subset. Cancer 94 (2): 459-69, 2002. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Oberlin O, et al.: Synovial sarcoma in children and adolescents: a critical reappraisal of staging investigations in relation to the rate of metastatic involvement at diagnosis. Eur J Cancer 48 (9): 1370-5, 2012. [PUBMED Abstract]
- Scheer M, Blank B, Bauer S, et al.: Synovial sarcoma disease characteristics and primary tumor sites differ between patient age groups: a report of the Cooperative Weichteilsarkom Studiengruppe (CWS). J Cancer Res Clin Oncol 146 (4): 953-960, 2020. [PUBMED Abstract]
- van de Rijn M, Barr FG, Collins MH, et al.: Absence of SYT-SSX fusion products in soft tissue tumors other than synovial sarcoma. Am J Clin Pathol 112 (1): 43-9, 1999. [PUBMED Abstract]
- Krsková L, Sumerauer D, Stejskalová E, et al.: A novel variant of SYT-SSX1 fusion gene in a case of spindle cell synovial sarcoma. Diagn Mol Pathol 16 (3): 179-83, 2007. [PUBMED Abstract]
- Su L, Sampaio AV, Jones KB, et al.: Deconstruction of the SS18-SSX fusion oncoprotein complex: insights into disease etiology and therapeutics. Cancer Cell 21 (3): 333-47, 2012. [PUBMED Abstract]
- Arnold MA, Arnold CA, Li G, et al.: A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol 44 (5): 881-7, 2013. [PUBMED Abstract]
- Vlenterie M, Ho VK, Kaal SE, et al.: Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer 113 (11): 1602-6, 2015. [PUBMED Abstract]
- Smolle MA, Parry M, Jeys L, et al.: Synovial sarcoma: Do children do better? Eur J Surg Oncol 45 (2): 254-260, 2019. [PUBMED Abstract]
- Okcu MF, Munsell M, Treuner J, et al.: Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol 21 (8): 1602-11, 2003. [PUBMED Abstract]
- Brecht IB, Ferrari A, Int-Veen C, et al.: Grossly-resected synovial sarcoma treated by the German and Italian Pediatric Soft Tissue Sarcoma Cooperative Groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer 46 (1): 11-7, 2006. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Healey JH, et al.: Pediatric and adolescent synovial sarcoma: multivariate analysis of prognostic factors and survival outcomes. Ann Surg Oncol 20 (1): 73-9, 2013. [PUBMED Abstract]
- Lagarde P, Przybyl J, Brulard C, et al.: Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. J Clin Oncol 31 (5): 608-15, 2013. [PUBMED Abstract]
- Stegmaier S, Leuschner I, Poremba C, et al.: The prognostic impact of SYT-SSX fusion type and histological grade in pediatric patients with synovial sarcoma treated according to the CWS (Cooperative Weichteilsarkom Studie) trials. Pediatr Blood Cancer 64 (1): 89-95, 2017. [PUBMED Abstract]
- Scheer M, Dantonello T, Hallmen E, et al.: Primary Metastatic Synovial Sarcoma: Experience of the CWS Study Group. Pediatr Blood Cancer 63 (7): 1198-206, 2016. [PUBMED Abstract]
- Orbach D, Mosseri V, Pissaloux D, et al.: Genomic complexity in pediatric synovial sarcomas (Synobio study): the European pediatric soft tissue sarcoma group (EpSSG) experience. Cancer Med 7 (4): 1384-1393, 2018. [PUBMED Abstract]
- Trassard M, Le Doussal V, Hacène K, et al.: Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 19 (2): 525-34, 2001. [PUBMED Abstract]
- Guillou L, Benhattar J, Bonichon F, et al.: Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 22 (20): 4040-50, 2004. [PUBMED Abstract]
- Ferrari A, Gronchi A, Casanova M, et al.: Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 101 (3): 627-34, 2004. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Ferrari A, Chi YY, De Salvo GL, et al.: Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children's Oncology Group. Eur J Cancer 78: 1-6, 2017. [PUBMED Abstract]
- McGrory JE, Pritchard DJ, Arndt CA, et al.: Nonrhabdomyosarcoma soft tissue sarcomas in children. The Mayo Clinic experience. Clin Orthop (374): 247-58, 2000. [PUBMED Abstract]
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al.: Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 17 (1): 150-7, 1999. [PUBMED Abstract]
- Koscielniak E, Harms D, Henze G, et al.: Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17 (12): 3706-19, 1999. [PUBMED Abstract]
- Pappo AS, Devidas M, Jenkins J, et al.: Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol 23 (18): 4031-8, 2005. [PUBMED Abstract]
- Cecchetto G, Alaggio R, Dall'Igna P, et al.: Localized unresectable non-rhabdo soft tissue sarcomas of the extremities in pediatric age: results from the Italian studies. Cancer 104 (9): 2006-12, 2005. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Brennan B, Stevens M, Kelsey A, et al.: Synovial sarcoma in childhood and adolescence: a retrospective series of 77 patients registered by the Children's Cancer and Leukaemia Group between 1991 and 2006. Pediatr Blood Cancer 55 (1): 85-90, 2010. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Raney RB: Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol Oncol 27 (4): 207-11, 2005. [PUBMED Abstract]
- Orbach D, Mc Dowell H, Rey A, et al.: Sparing strategy does not compromise prognosis in pediatric localized synovial sarcoma: experience of the International Society of Pediatric Oncology, Malignant Mesenchymal Tumors (SIOP-MMT) Working Group. Pediatr Blood Cancer 57 (7): 1130-6, 2011. [PUBMED Abstract]
- Ladenstein R, Treuner J, Koscielniak E, et al.: Synovial sarcoma of childhood and adolescence. Report of the German CWS-81 study. Cancer 71 (11): 3647-55, 1993. [PUBMED Abstract]
- Venkatramani R, Xue W, Randall RL, et al.: Synovial Sarcoma in Children, Adolescents, and Young Adults: A Report From the Children's Oncology Group ARST0332 Study. J Clin Oncol 39 (35): 3927-3937, 2021. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Brennan B, et al.: Synovial sarcoma in children and adolescents: the European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Ann Oncol 26 (3): 567-72, 2015. [PUBMED Abstract]
- Scheer M, Hallmen E, Vokuhl C, et al.: Pre-operative radiotherapy is associated with superior local relapse-free survival in advanced synovial sarcoma. J Cancer Res Clin Oncol 149 (5): 1717-1731, 2023. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Scheer M, Dantonello T, Hallmen E, et al.: Synovial Sarcoma Recurrence in Children and Young Adults. Ann Surg Oncol 23 (Suppl 5): 618-626, 2016. [PUBMED Abstract]
- Ferrari A, Orbach D, Bergamaschi L, et al.: Treatment at relapse for synovial sarcoma of children and adolescents: A multi-institutional European retrospective analysis. Pediatr Blood Cancer 71 (7): e31038, 2024. [PUBMED Abstract]
- Dhakal S, Corbin KS, Milano MT, et al.: Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 82 (2): 940-5, 2012. [PUBMED Abstract]
- Lai JP, Robbins PF, Raffeld M, et al.: NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol 25 (6): 854-8, 2012. [PUBMED Abstract]
- Robbins PF, Kassim SH, Tran TL, et al.: A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 21 (5): 1019-27, 2015. [PUBMED Abstract]
- D'Angelo SP, Melchiori L, Merchant MS, et al.: Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 c259T Cells in Synovial Sarcoma. Cancer Discov 8 (8): 944-957, 2018. [PUBMED Abstract]
- D'Angelo SP, Araujo DM, Abdul Razak AR, et al.: Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. Lancet 403 (10435): 1460-1471, 2024. [PUBMED Abstract]
- Chbani L, Guillou L, Terrier P, et al.: Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol 131 (2): 222-7, 2009. [PUBMED Abstract]
- Hornick JL, Dal Cin P, Fletcher CD: Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 33 (4): 542-50, 2009. [PUBMED Abstract]
- Knutson SK, Warholic NM, Wigle TJ, et al.: Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A 110 (19): 7922-7, 2013. [PUBMED Abstract]
- Guzzetta AA, Montgomery EA, Lyu H, et al.: Epithelioid sarcoma: one institution's experience with a rare sarcoma. J Surg Res 177 (1): 116-22, 2012. [PUBMED Abstract]
- Hawkins DS, Spunt SL, Skapek SX, et al.: Children's Oncology Group's 2013 blueprint for research: Soft tissue sarcomas. Pediatr Blood Cancer 60 (6): 1001-8, 2013. [PUBMED Abstract]
- Sparber-Sauer M, Koscielniak E, Vokuhl C, et al.: Epithelioid sarcoma in children, adolescents, and young adults: Localized, primary metastatic and relapsed disease. Treatment results of five Cooperative Weichteilsarkom Studiengruppe (CWS) trials and one registry. Pediatr Blood Cancer 66 (9): e27879, 2019. [PUBMED Abstract]
- Spunt SL, Francotte N, De Salvo GL, et al.: Clinical features and outcomes of young patients with epithelioid sarcoma: an analysis from the Children's Oncology Group and the European paediatric soft tissue Sarcoma Study Group prospective clinical trials. Eur J Cancer 112: 98-106, 2019. [PUBMED Abstract]
- Casanova M, Ferrari A, Collini P, et al.: Epithelioid sarcoma in children and adolescents: a report from the Italian Soft Tissue Sarcoma Committee. Cancer 106 (3): 708-17, 2006. [PUBMED Abstract]
- Gounder M, Schöffski P, Jones RL, et al.: Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol 21 (11): 1423-1432, 2020. [PUBMED Abstract]
- Orbach D, Brennan B, Casanova M, et al.: Paediatric and adolescent alveolar soft part sarcoma: A joint series from European cooperative groups. Pediatr Blood Cancer 60 (11): 1826-32, 2013. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Wang HW, Qin XJ, Yang WJ, et al.: Alveolar soft part sarcoma of the oral and maxillofacial region: clinical analysis in a series of 18 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 119 (4): 396-401, 2015. [PUBMED Abstract]
- Kayton ML, Meyers P, Wexler LH, et al.: Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg 41 (1): 187-93, 2006. [PUBMED Abstract]
- Sparber-Sauer M, Seitz G, von Kalle T, et al.: Alveolar soft-part sarcoma: Primary metastatic disease and metastatic relapse occurring during long-term follow-up: Treatment results of four Cooperative Weichteilsarkom Studiengruppe (CWS) trials and one registry. Pediatr Blood Cancer 65 (12): e27405, 2018. [PUBMED Abstract]
- Flores RJ, Harrison DJ, Federman NC, et al.: Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer 65 (5): e26953, 2018. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Lieberman PH, Brennan MF, Kimmel M, et al.: Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer 63 (1): 1-13, 1989. [PUBMED Abstract]
- Casanova M, Ferrari A, Bisogno G, et al.: Alveolar soft part sarcoma in children and adolescents: A report from the Soft-Tissue Sarcoma Italian Cooperative Group. Ann Oncol 11 (11): 1445-9, 2000. [PUBMED Abstract]
- Pennacchioli E, Fiore M, Collini P, et al.: Alveolar soft part sarcoma: clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol 17 (12): 3229-33, 2010. [PUBMED Abstract]
- Wilky BA, Trucco MM, Subhawong TK, et al.: Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol 20 (6): 837-848, 2019. [PUBMED Abstract]
- Stacchiotti S, Negri T, Zaffaroni N, et al.: Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol 22 (7): 1682-90, 2011. [PUBMED Abstract]
- Jagodzińska-Mucha P, Świtaj T, Kozak K, et al.: Long-term results of therapy with sunitinib in metastatic alveolar soft part sarcoma. Tumori 103 (3): 231-235, 2017. [PUBMED Abstract]
- Cohen JW, Widemann BC, Derdak J, et al.: Cediranib phase-II study in children with metastatic alveolar soft-part sarcoma (ASPS). Pediatr Blood Cancer 66 (12): e27987, 2019. [PUBMED Abstract]
- Judson I, Morden JP, Kilburn L, et al.: Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol 20 (7): 1023-1034, 2019. [PUBMED Abstract]
- Kummar S, Allen D, Monks A, et al.: Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 31 (18): 2296-302, 2013. [PUBMED Abstract]
- Kim M, Kim TM, Keam B, et al.: A Phase II Trial of Pazopanib in Patients with Metastatic Alveolar Soft Part Sarcoma. Oncologist 24 (1): 20-e29, 2019. [PUBMED Abstract]
- Stacchiotti S, Mir O, Le Cesne A, et al.: Activity of Pazopanib and Trabectedin in Advanced Alveolar Soft Part Sarcoma. Oncologist 23 (1): 62-70, 2018. [PUBMED Abstract]
- Chen AP, Sharon E, O'Sullivan-Coyne G, et al.: Atezolizumab for Advanced Alveolar Soft Part Sarcoma. N Engl J Med 389 (10): 911-921, 2023. [PUBMED Abstract]
- Roozendaal KJ, de Valk B, ten Velden JJ, et al.: Alveolar soft-part sarcoma responding to interferon alpha-2b. Br J Cancer 89 (2): 243-5, 2003. [PUBMED Abstract]
- Conde N, Cruz O, Albert A, et al.: Antiangiogenic treatment as a pre-operative management of alveolar soft-part sarcoma. Pediatr Blood Cancer 57 (6): 1071-3, 2011. [PUBMED Abstract]
- Meis-Kindblom JM: Clear cell sarcoma of tendons and aponeuroses: a historical perspective and tribute to the man behind the entity. Adv Anat Pathol 13 (6): 286-92, 2006. [PUBMED Abstract]
- Dim DC, Cooley LD, Miranda RN: Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med 131 (1): 152-6, 2007. [PUBMED Abstract]
- Blazer DG, Lazar AJ, Xing Y, et al.: Clinical outcomes of molecularly confirmed clear cell sarcoma from a single institution and in comparison with data from the Surveillance, Epidemiology, and End Results registry. Cancer 115 (13): 2971-9, 2009. [PUBMED Abstract]
- Coindre JM, Hostein I, Terrier P, et al.: Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer 107 (5): 1055-64, 2006. [PUBMED Abstract]
- Fujimura Y, Siddique H, Lee L, et al.: EWS-ATF-1 chimeric protein in soft tissue clear cell sarcoma associates with CREB-binding protein and interferes with p53-mediated trans-activation function. Oncogene 20 (46): 6653-9, 2001. [PUBMED Abstract]
- Hisaoka M, Ishida T, Kuo TT, et al.: Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol 32 (3): 452-60, 2008. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Clear cell sarcoma of tendons and aponeuroses in pediatric patients: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 94 (12): 3269-76, 2002. [PUBMED Abstract]
- Karita M, Tsuchiya H, Yamamoto N, et al.: Caffeine-potentiated chemotherapy for clear cell sarcoma: a report of five cases. Int J Clin Oncol 18 (1): 33-7, 2013. [PUBMED Abstract]
- Schöffski P, Wozniak A, Stacchiotti S, et al.: Activity and safety of crizotinib in patients with advanced clear-cell sarcoma with MET alterations: European Organization for Research and Treatment of Cancer phase II trial 90101 'CREATE'. Ann Oncol 28 (12): 3000-3008, 2017. [PUBMED Abstract]
- Tsuneyoshi M, Enjoji M, Iwasaki H, et al.: Extraskeletal myxoid chondrosarcoma--a clinicopathologic and electron microscopic study. Acta Pathol Jpn 31 (3): 439-47, 1981. [PUBMED Abstract]
- Hachitanda Y, Tsuneyoshi M, Daimaru Y, et al.: Extraskeletal myxoid chondrosarcoma in young children. Cancer 61 (12): 2521-6, 1988. [PUBMED Abstract]
- Enzinger FM, Shiraki M: Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol 3 (3): 421-35, 1972. [PUBMED Abstract]
- McGrory JE, Rock MG, Nascimento AG, et al.: Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat Res (382): 185-90, 2001. [PUBMED Abstract]
- Drilon AD, Popat S, Bhuchar G, et al.: Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer 113 (12): 3364-71, 2008. [PUBMED Abstract]
- Hisaoka M, Ishida T, Imamura T, et al.: TFG is a novel fusion partner of NOR1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer 40 (4): 325-8, 2004. [PUBMED Abstract]
- Stacchiotti S, Pantaleo MA, Astolfi A, et al.: Activity of sunitinib in extraskeletal myxoid chondrosarcoma. Eur J Cancer 50 (9): 1657-64, 2014. [PUBMED Abstract]
- Leavey PJ, Laack NN, Krailo MD, et al.: Phase III Trial Adding Vincristine-Topotecan-Cyclophosphamide to the Initial Treatment of Patients With Nonmetastatic Ewing Sarcoma: A Children's Oncology Group Report. J Clin Oncol 39 (36): 4029-4038, 2021. [PUBMED Abstract]
- Leuschner I, Radig K, Harms D: Desmoplastic small round cell tumor. Semin Diagn Pathol 13 (3): 204-12, 1996. [PUBMED Abstract]
- Kushner BH, LaQuaglia MP, Wollner N, et al.: Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 14 (5): 1526-31, 1996. [PUBMED Abstract]
- Saab R, Khoury JD, Krasin M, et al.: Desmoplastic small round cell tumor in childhood: the St. Jude Children's Research Hospital experience. Pediatr Blood Cancer 49 (3): 274-9, 2007. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Hayes-Jordan A, LaQuaglia MP, Modak S: Management of desmoplastic small round cell tumor. Semin Pediatr Surg 25 (5): 299-304, 2016. [PUBMED Abstract]
- Arora VC, Price AP, Fleming S, et al.: Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol 43 (1): 93-102, 2013. [PUBMED Abstract]
- Gerald WL, Ladanyi M, de Alava E, et al.: Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol 16 (9): 3028-36, 1998. [PUBMED Abstract]
- Slotkin EK, Bowman AS, Levine MF, et al.: Comprehensive Molecular Profiling of Desmoplastic Small Round Cell Tumor. Mol Cancer Res 19 (7): 1146-1155, 2021. [PUBMED Abstract]
- Chow WA, Yee JK, Tsark W, et al.: Recurrent secondary genomic alterations in desmoplastic small round cell tumors. BMC Med Genet 21 (1): 101, 2020. [PUBMED Abstract]
- Lal DR, Su WT, Wolden SL, et al.: Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 40 (1): 251-5, 2005. [PUBMED Abstract]
- Philippe-Chomette P, Kabbara N, Andre N, et al.: Desmoplastic small round cell tumors with EWS-WT1 fusion transcript in children and young adults. Pediatr Blood Cancer 58 (6): 891-7, 2012. [PUBMED Abstract]
- Sedig L, Geiger J, Mody R, et al.: Paratesticular desmoplastic small round cell tumors: A case report and review of the literature. Pediatr Blood Cancer 64 (12): , 2017. [PUBMED Abstract]
- Subbiah V, Lamhamedi-Cherradi SE, Cuglievan B, et al.: Multimodality Treatment of Desmoplastic Small Round Cell Tumor: Chemotherapy and Complete Cytoreductive Surgery Improve Patient Survival. Clin Cancer Res 24 (19): 4865-4873, 2018. [PUBMED Abstract]
- Schwarz RE, Gerald WL, Kushner BH, et al.: Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol 5 (5): 416-22, 1998 Jul-Aug. [PUBMED Abstract]
- Goodman KA, Wolden SL, La Quaglia MP, et al.: Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys 54 (1): 170-6, 2002. [PUBMED Abstract]
- Osborne EM, Briere TM, Hayes-Jordan A, et al.: Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol 119 (1): 40-4, 2016. [PUBMED Abstract]
- Atallah V, Honore C, Orbach D, et al.: Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys 95 (4): 1244-53, 2016. [PUBMED Abstract]
- Hayes-Jordan AA, Coakley BA, Green HL, et al.: Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann Surg Oncol 25 (4): 872-877, 2018. [PUBMED Abstract]
- Scalabre A, Philippe-Chomette P, Passot G, et al.: Cytoreductive surgery and hyperthermic intraperitoneal perfusion with chemotherapy in children with peritoneal tumor spread: A French nationwide study over 14 years. Pediatr Blood Cancer 65 (4): , 2018. [PUBMED Abstract]
- Honoré C, Atallah V, Mir O, et al.: Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS One 12 (2): e0171639, 2017. [PUBMED Abstract]
- Stiles ZE, Murphy AJ, Anghelescu DL, et al.: Desmoplastic Small Round Cell Tumor: Long-Term Complications After Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 27 (1): 171-178, 2020. [PUBMED Abstract]
- Cook RJ, Wang Z, Arora M, et al.: Clinical outcomes of patients with desmoplastic small round cell tumor of the peritoneum undergoing autologous HCT: a CIBMTR retrospective analysis. Bone Marrow Transplant 47 (11): 1455-8, 2012. [PUBMED Abstract]
- Tarek N, Hayes-Jordan A, Salvador L, et al.: Recurrent desmoplastic small round cell tumor responding to an mTOR inhibitor containing regimen. Pediatr Blood Cancer 65 (1): , 2018. [PUBMED Abstract]
- Kodet R, Newton WA, Sachs N, et al.: Rhabdoid tumors of soft tissues: a clinicopathologic study of 26 cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum Pathol 22 (7): 674-84, 1991. [PUBMED Abstract]
- Biegel JA, Zhou JY, Rorke LB, et al.: Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59 (1): 74-9, 1999. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Lee RS, Stewart C, Carter SL, et al.: A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122 (8): 2983-8, 2012. [PUBMED Abstract]
- Teplick A, Kowalski M, Biegel JA, et al.: Educational paper: screening in cancer predisposition syndromes: guidelines for the general pediatrician. Eur J Pediatr 170 (3): 285-94, 2011. [PUBMED Abstract]
- Mitchell SG, Pencheva B, Porter CC: Germline Genetics and Childhood Cancer: Emerging Cancer Predisposition Syndromes and Psychosocial Impacts. Curr Oncol Rep 21 (10): 85, 2019. [PUBMED Abstract]
- Foulkes WD, Kamihara J, Evans DGR, et al.: Cancer Surveillance in Gorlin Syndrome and Rhabdoid Tumor Predisposition Syndrome. Clin Cancer Res 23 (12): e62-e67, 2017. [PUBMED Abstract]
- Morgan KM, Siow VS, Strotmeyer S, et al.: Characteristics and Outcomes in Pediatric Non-Central Nervous System Malignant Rhabdoid Tumors: A Report from the National Cancer Database. Ann Surg Oncol 29 (1): 671-678, 2022. [PUBMED Abstract]
- Sultan I, Qaddoumi I, Rodríguez-Galindo C, et al.: Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer 54 (1): 35-40, 2010. [PUBMED Abstract]
- Nemes K, Bens S, Kachanov D, et al.: Clinical and genetic risk factors define two risk groups of extracranial malignant rhabdoid tumours (eMRT/RTK). Eur J Cancer 142: 112-122, 2021. [PUBMED Abstract]
- Puri DR, Meyers PA, Kraus DH, et al.: Radiotherapy in the multimodal treatment of extrarenal extracranial malignant rhabdoid tumors. Pediatr Blood Cancer 50 (1): 167-9, 2008. [PUBMED Abstract]
- Madigan CE, Armenian SH, Malogolowkin MH, et al.: Extracranial malignant rhabdoid tumors in childhood: the Childrens Hospital Los Angeles experience. Cancer 110 (9): 2061-6, 2007. [PUBMED Abstract]
- Bourdeaut F, Fréneaux P, Thuille B, et al.: Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer 51 (3): 363-8, 2008. [PUBMED Abstract]
- Wetmore C, Boyett J, Li S, et al.: Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol 17 (6): 882-8, 2015. [PUBMED Abstract]
- Folpe A, Inwards C, eds.: Bone and Soft Tissue Pathology: A Volume in the Foundations in Diagnostic Pathology. WB Saunders Co, 2010.
- Martignoni G, Pea M, Reghellin D, et al.: Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med 134 (1): 33-40, 2010. [PUBMED Abstract]
- Bissler JJ, McCormack FX, Young LR, et al.: Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358 (2): 140-51, 2008. [PUBMED Abstract]
- Davies DM, Johnson SR, Tattersfield AE, et al.: Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 358 (2): 200-3, 2008. [PUBMED Abstract]
- Agaram NP, Sung YS, Zhang L, et al.: Dichotomy of Genetic Abnormalities in PEComas With Therapeutic Implications. Am J Surg Pathol 39 (6): 813-25, 2015. [PUBMED Abstract]
- Armah HB, Parwani AV: Perivascular epithelioid cell tumor. Arch Pathol Lab Med 133 (4): 648-54, 2009. [PUBMED Abstract]
- Alaggio R, Cecchetto G, Martignoni G, et al.: Malignant perivascular epithelioid cell tumor in children: description of a case and review of the literature. J Pediatr Surg 47 (6): e31-40, 2012. [PUBMED Abstract]
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al.: Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 28 (5): 835-40, 2010. [PUBMED Abstract]
- Wagner AJ, Ravi V, Riedel RF, et al.: nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors. J Clin Oncol 39 (33): 3660-3670, 2021. [PUBMED Abstract]
- Laetsch TW, Roy A, Xu L, et al.: Undifferentiated Sarcomas in Children Harbor Clinically Relevant Oncogenic Fusions and Gene Copy-Number Alterations: A Report from the Children's Oncology Group. Clin Cancer Res 24 (16): 3888-3897, 2018. [PUBMED Abstract]
- Randall RL, Albritton KH, Ferney BJ, et al.: Malignant fibrous histiocytoma of soft tissue: an abandoned diagnosis. Am J Orthop 33 (12): 602-8, 2004. [PUBMED Abstract]
- Fletcher CDM, Bridge JA, Hogendoorn P, et al., eds.: WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press, 2013.
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
- Daw NC, Billups CA, Pappo AS, et al.: Malignant fibrous histiocytoma and other fibrohistiocytic tumors in pediatric patients: the St. Jude Children's Research Hospital experience. Cancer 97 (11): 2839-47, 2003. [PUBMED Abstract]
- Bjerkehagen B, Smeland S, Walberg L, et al.: Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol 47 (8): 1475-82, 2008. [PUBMED Abstract]
- Le Guellec S, Chibon F, Ouali M, et al.: Are peripheral purely undifferentiated pleomorphic sarcomas with MDM2 amplification dedifferentiated liposarcomas? Am J Surg Pathol 38 (3): 293-304, 2014. [PUBMED Abstract]
- Tawbi HA, Burgess M, Bolejack V, et al.: Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 18 (11): 1493-1501, 2017. [PUBMED Abstract]
- Sloan EA, Gupta R, Koelsche C, et al.: Intracranial mesenchymal tumors with FET-CREB fusion are composed of at least two epigenetic subgroups distinct from meningioma and extracranial sarcomas. Brain Pathol 32 (4): e13037, 2022. [PUBMED Abstract]
- Sloan EA, Chiang J, Villanueva-Meyer JE, et al.: Intracranial mesenchymal tumor with FET-CREB fusion-A unifying diagnosis for the spectrum of intracranial myxoid mesenchymal tumors and angiomatoid fibrous histiocytoma-like neoplasms. Brain Pathol 31 (4): e12918, 2021. [PUBMED Abstract]
Treatment of Vascular Tumors
Vascular tumors vary from hemangiomas, which are always considered benign, to angiosarcomas, which are highly malignant.[1] Malignant vascular tumors include the following subtypes:
Epithelioid Hemangioendothelioma NOS
Incidence and outcome
Epithelioid hemangioendothelioma was first described in soft tissue by Weiss and Enzinger in 1982. These tumors can occur in younger patients, but the peak incidence is in the fourth and fifth decades of life. The number of pediatric patients reported in the literature is limited.
Epithelioid hemangioendotheliomas can have an indolent or very aggressive course, with an overall survival rate of 73% at 5 years. There are case reports of patients with untreated multiple lesions who have a very benign course. However, other patients have a very aggressive course. Some pathologists have tried to stratify patients to evaluate risks and adjust treatment, but more research is needed.[2-8]
A multi-institutional case series reported on 24 patients aged 2 to 26 years with epithelioid hemangioendotheliomas.[9][Level of evidence C2] Most patients presented with multiorgan disease. Progression was seen in 63% of patients, with a mean time to progression of 18.4 months (range, 0–72 months).
The presence of effusions, tumor size larger than 3 cm, and a high mitotic index (>3 mitoses/50 high-power fields) have been associated with unfavorable outcomes.[4]
Clinical presentation and diagnostic evaluation
Common sites of involvement are liver alone (21%), liver plus lung (18%), lung alone (12%), and bone alone (14%).[4,10,11] Clinical presentation depends on the site of involvement, as follows:
- Liver: Hepatic nodules have central vascularity on ultrasound, contrast-enhancing lesions by computed tomography, and low T1 signal and moderate T2 signal on magnetic resonance imaging. These may be incidental findings in asymptomatic patients, but most patients commonly present with signs or symptoms of cholestasis, including pruritus, jaundice, or scleral icterus.
- Lung: Pulmonary epithelioid hemangioendothelioma may be an asymptomatic finding on chest x-ray or be associated with pleuritic pain, hemoptysis, anemia, and fibrosis.
- Bone: Bone metastasis may be associated with pathological fracture. On x-rays, they are well-defined osteolytic lesions and can be multiple or solitary.
- Soft tissue: Thirty percent of soft tissue cases are associated with metastases. When present, metastatic disease can be very aggressive and have a limited response to chemotherapy.
- Skin: Cutaneous lesions can be raised and nodular or can be warm, red-brown plaques.
Genomic alterations and histopathological features
WWTR1::CAMTA1 gene fusions have been found in most patients. Less commonly, YAP1::TFE3 gene fusions have been reported.[2] These gene fusions are not directly targetable with current medications. Monoclonality has been described in multiple liver lesions, suggesting a metastatic process.
Histologically, these lesions are characterized as epithelioid lesions arranged in nests, strands, and trabecular patterns, with infrequent vascular spaces. Features that may be associated with aggressive clinical behavior include cellular atypia, one or more mitoses per 10 high-power fields, an increased proportion of spindled cells, focal necrosis, and metaplastic bone formation.[4]
Treatment of epithelioid hemangioendothelioma
Treatment options for epithelioid hemangioendothelioma include the following:
- Observation.
- Surgery.
- Immunotherapy.
- Targeted therapy.
- Chemotherapy.
- Radiation therapy.
For indolent cases, observation is warranted. Surgery is performed when resection is possible. Liver transplant has been used with aggressive liver lesions, both with and without metastases.[4,12-14]
For more aggressive cases, several different drugs have been used, including interferon, thalidomide, sorafenib, pazopanib, and sirolimus.[12,15,16] The most aggressive cases are treated with angiosarcoma-type chemotherapy.
A multi-institutional case series reported on 24 patients aged 2 to 26 years with epithelioid hemangioendothelioma.[9][Level of evidence C2]
- Three patients who were treated with sirolimus had stable disease or partial responses for more than 2.5 years.
A report from 2020 that investigated sirolimus treatment in children aimed to add to the previous experience of sirolimus in adults. A retrospective review identified six pediatric patients with disseminated epithelioid hemangioendothelioma who were treated with sirolimus.[17]
- Four of the six patients demonstrated partial responses or disease stabilization.
A report from the European paediatric Soft Tissue Sarcoma Study Group analyzed ten patients with localized disease and one patient with metastatic disease from two studies.[18] The median age was 14.3 years (range, 9.0–18.8 years). Local therapy was initial primary surgery in seven patients, and five patients received systemic therapy. No patients received radiation therapy.
- After a median follow-up of 50 months (range, 6–176 months), nine patients remained alive and off therapy and two patients died.
- The 5-year progression-free survival rate was 77.1% (95% confidence interval [CI], 34.5%–93.9%).
- The 5-year overall survival rate was 74.1% (95% CI, 28.1%–93.0%).
Patients or families who desire additional disease-directed therapy should consider entering trials of novel therapeutic approaches because no standard agents have demonstrated clinically significant activity.
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Treatment options under clinical evaluation for epithelioid hemangioendothelioma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Angiosarcoma
Incidence and clinical presentation
Angiosarcomas are rare (accounting for 2% of sarcomas), aggressive, vascular tumors that can arise in any part of the body but is more common in soft tissues. Angiosarcoma has an estimated incidence of 2 cases per 1 million people. In the United States, it affects approximately 600 people annually, who are typically aged 60 to 70 years.[19]
Angiosarcomas are extremely rare in children. It is unclear if the pathophysiology of angiosarcomas in children differs from that of angiosarcomas in adults. Cases have been reported in neonates and toddlers, with presentation of multiple cutaneous lesions and liver lesions, some of which are GLUT1 positive.[20-23] Most angiosarcomas involve the skin and superficial soft tissue, although the liver, spleen, and lung can be affected; bone is rarely affected.
Nomenclature of these liver lesions has been difficult and confusing with use of outdated terminology proposed in 1971 (e.g., type I hemangioendothelioma: infantile hemangioma; type II hemangioendothelioma: low-grade angiosarcoma; type III hemangioendothelioma: high-grade angiosarcoma).[21] A report of eight cases of liver angiosarcomas in children highlighted the misuse of the term hemangioendothelioma and the importance of early diagnosis and treatment of these tumors.[24]
Risk factors
Established risk factors include the following:[25]
- Vinyl chloride exposure.
- Radiation exposure.
- Chronic lymphedema from any cause, including Stewart-Treves syndrome.
Genomic alterations and histopathological features
Angiosarcomas are largely aneuploid tumors. The rare cases of angiosarcoma that arise from benign lesions such as hemangiomas have a distinct pathway that needs to be investigated. MYC amplification is seen in radiation-induced angiosarcoma. KDR variants and FLT4 amplifications have been seen with a frequency of less than 50%.[25]
Histopathological diagnosis can be very difficult because there can be areas of varied atypia. A common feature of angiosarcoma is an irregular network of channels in a dissective pattern along dermal collagen bundles. There is varied cellular shape, size, mitosis, endothelial multilayering, and papillary formation. Epithelioid cells can also be present. Necrosis and hemorrhage are common. Tumors stain for factor VIII, CD31, and CD34. Some liver lesions can mimic infantile hemangiomas and have focal GLUT1 positivity.[21]
Treatment of angiosarcoma
Treatment options for angiosarcoma include the following:
Surgery
Localized disease can be cured by aggressive surgery. Complete surgical excision appears to be crucial for the long-term survival of patients with angiosarcomas and lymphangiosarcomas, despite evidence of tumor shrinkage in some patients who were treated with local or systemic therapy.[22,26-28] Data on liver transplant for localized angiosarcomas are limited.[29][Level of evidence C1]
Evidence (surgery):
- A review of 222 patients (median age, 62 years; range, 15–90 years) reported the following:[28]
- An overall disease-specific survival (DSS) rate of 38% at 5 years.
- The 5-year DSS rate was 44% in 138 patients with localized, resected tumors but only 16% in 43 patients with metastases at diagnosis.
- One case report suggested that liver transplant may contribute to prolonged disease-free survival.[30][Level of evidence C2]
Radiation therapy
Localized disease, especially cutaneous angiosarcomas, can be treated with radiation therapy or combined chemotherapy (e.g., paclitaxel) and radiation therapy.[31] Most of these reported cases are in adults.[32] When radiation is used, the doses are high (50–70 Gy), the cutaneous volumes are extensive because of the infiltrating nature of the disease, and regional (draining) nodes are often included, even if clinically negative.[33,34] Because of these factors, radiation therapy is rarely used to treat children.
Surgery, chemotherapy, and radiation therapy
Multimodal treatment with surgery, systemic chemotherapy, and radiation therapy is used for metastatic disease, although it is rarely curative.[34,35] Disease control is the objective in patients with metastatic angiosarcomas. Published progression-free survival is between 3 months and 7 months,[36] and the median overall survival (OS) is 14 to 18 months.[37] In both adults and children, the 5-year OS rates are between 20% and 35%.[22,23,38]
One child who was diagnosed with angiosarcoma secondary to malignant transformation from infantile hemangioma responded to treatment with bevacizumab (a monoclonal antibody against vascular endothelial growth factor) combined with systemic chemotherapy.[20,35]
Biologic agents that inhibit angiogenesis have shown activity in adults with angiosarcomas.[21,38]
There is one case report of a pediatric patient with metastatic cardiac angiosarcoma who was successfully treated with conventional chemotherapy, radiation, surgery, and targeted therapies, including pazopanib.[39]
Palliative care
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Treatment options under clinical evaluation for angiosarcoma
Patients or families who desire additional disease-directed therapy should consider entering trials of novel therapeutic approaches because no standard agents have demonstrated clinically significant activity.
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Coffin CM, Dehner LP: Vascular tumors in children and adolescents: a clinicopathologic study of 228 tumors in 222 patients. Pathol Annu 28 Pt 1: 97-120, 1993. [PUBMED Abstract]
- Mehrabi A, Kashfi A, Fonouni H, et al.: Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 107 (9): 2108-21, 2006. [PUBMED Abstract]
- Haro A, Saitoh G, Tamiya S, et al.: Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer 6 (4): 544-7, 2015. [PUBMED Abstract]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al.: Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 8 (2): 259, 2014. [PUBMED Abstract]
- Dong K, Wang XX, Feng JL, et al.: Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 8 (9): 11015-23, 2015. [PUBMED Abstract]
- Adams DM, Hammill A: Other vascular tumors. Semin Pediatr Surg 23 (4): 173-7, 2014. [PUBMED Abstract]
- Xiao Y, Wang C, Song Y, et al.: Primary epithelioid hemangioendothelioma of the kidney: the first case report in a child and literature review. Urology 82 (4): 925-7, 2013. [PUBMED Abstract]
- Reich S, Ringe H, Uhlenberg B, et al.: Epithelioid hemangioendothelioma of the lung presenting with pneumonia and heart rhythm disturbances in a teenage girl. J Pediatr Hematol Oncol 32 (4): 274-6, 2010. [PUBMED Abstract]
- Cournoyer E, Al-Ibraheemi A, Engel E, et al.: Clinical characterization and long-term outcomes in pediatric epithelioid hemangioendothelioma. Pediatr Blood Cancer 67 (2): e28045, 2020. [PUBMED Abstract]
- Daller JA, Bueno J, Gutierrez J, et al.: Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 34 (1): 98-105; discussion 105-6, 1999. [PUBMED Abstract]
- Ackermann O, Fabre M, Franchi S, et al.: Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr 53 (6): 615-9, 2011. [PUBMED Abstract]
- Raheja A, Suri A, Singh S, et al.: Multimodality management of a giant skull base hemangioendothelioma of the sphenopetroclival region. J Clin Neurosci 22 (9): 1495-8, 2015. [PUBMED Abstract]
- Ahmad N, Adams DM, Wang J, et al.: Hepatic epithelioid hemangioendothelioma in a patient with hemochromatosis. J Natl Compr Canc Netw 12 (9): 1203-7, 2014. [PUBMED Abstract]
- Otte JB, Zimmerman A: The role of liver transplantation for pediatric epithelioid hemangioendothelioma. Pediatr Transplant 14 (3): 295-7, 2010. [PUBMED Abstract]
- Stacchiotti S, Provenzano S, Dagrada G, et al.: Sirolimus in Advanced Epithelioid Hemangioendothelioma: A Retrospective Case-Series Analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol 23 (9): 2735-44, 2016. [PUBMED Abstract]
- Semenisty V, Naroditsky I, Keidar Z, et al.: Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 15: 402, 2015. [PUBMED Abstract]
- Engel ER, Cournoyer E, Adams DM, et al.: A Retrospective Review of the Use of Sirolimus for Pediatric Patients With Epithelioid Hemangioendothelioma. J Pediatr Hematol Oncol 42 (8): e826-e829, 2020. [PUBMED Abstract]
- Orbach D, Van Noesel MM, Brennan B, et al.: Epithelioid hemangioendothelioma in children: The European Pediatric Soft Tissue Sarcoma Study Group experience. Pediatr Blood Cancer 69 (10): e29882, 2022. [PUBMED Abstract]
- Cioffi A, Reichert S, Antonescu CR, et al.: Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am 27 (5): 975-88, 2013. [PUBMED Abstract]
- Jeng MR, Fuh B, Blatt J, et al.: Malignant transformation of infantile hemangioma to angiosarcoma: response to chemotherapy with bevacizumab. Pediatr Blood Cancer 61 (11): 2115-7, 2014. [PUBMED Abstract]
- Dehner LP, Ishak KG: Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol 92 (2): 101-11, 1971. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol 39 (2): 109-14, 2002. [PUBMED Abstract]
- Deyrup AT, Miettinen M, North PE, et al.: Pediatric cutaneous angiosarcomas: a clinicopathologic study of 10 cases. Am J Surg Pathol 35 (1): 70-5, 2011. [PUBMED Abstract]
- Grassia KL, Peterman CM, Iacobas I, et al.: Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 64 (11): , 2017. [PUBMED Abstract]
- Elliott P, Kleinschmidt I: Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup Environ Med 54 (1): 14-8, 1997. [PUBMED Abstract]
- Lezama-del Valle P, Gerald WL, Tsai J, et al.: Malignant vascular tumors in young patients. Cancer 83 (8): 1634-9, 1998. [PUBMED Abstract]
- Fata F, O'Reilly E, Ilson D, et al.: Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 86 (10): 2034-7, 1999. [PUBMED Abstract]
- Lahat G, Dhuka AR, Hallevi H, et al.: Angiosarcoma: clinical and molecular insights. Ann Surg 251 (6): 1098-106, 2010. [PUBMED Abstract]
- Orlando G, Adam R, Mirza D, et al.: Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 95 (6): 872-7, 2013. [PUBMED Abstract]
- Aldén J, Baecklund F, Psaros Einberg A, et al.: Is primary hepatic angiosarcoma in children an indication for liver transplantation?-A single-centre experience and review of the literature. Pediatr Transplant 25 (8): e14095, 2021. [PUBMED Abstract]
- Roy A, Gabani P, Davis EJ, et al.: Concurrent paclitaxel and radiation therapy for the treatment of cutaneous angiosarcoma. Clin Transl Radiat Oncol 27: 114-120, 2021. [PUBMED Abstract]
- Sanada T, Nakayama H, Irisawa R, et al.: Clinical outcome and dose volume evaluation in patients who undergo brachytherapy for angiosarcoma of the scalp and face. Mol Clin Oncol 6 (3): 334-340, 2017. [PUBMED Abstract]
- Guadagnolo BA, Zagars GK, Araujo D, et al.: Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck 33 (5): 661-7, 2011. [PUBMED Abstract]
- Scott MT, Portnow LH, Morris CG, et al.: Radiation therapy for angiosarcoma: the 35-year University of Florida experience. Am J Clin Oncol 36 (2): 174-80, 2013. [PUBMED Abstract]
- Dickson MA, D'Adamo DR, Keohan ML, et al.: Phase II Trial of Gemcitabine and Docetaxel with Bevacizumab in Soft Tissue Sarcoma. Sarcoma 2015: 532478, 2015. [PUBMED Abstract]
- North PE, Waner M, Mizeracki A, et al.: A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol 137 (5): 559-70, 2001. [PUBMED Abstract]
- Boye E, Yu Y, Paranya G, et al.: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 107 (6): 745-52, 2001. [PUBMED Abstract]
- Ravi V, Patel S: Vascular sarcomas. Curr Oncol Rep 15 (4): 347-55, 2013. [PUBMED Abstract]
- Koo J, Knight-Perry J, Galambos C, et al.: Pediatric Metastatic Cardiac Angiosarcoma Successfully Treated With Multimodal Therapy: Case Report and Review of Literature. J Pediatr Hematol Oncol 43 (2): e203-e206, 2021. [PUBMED Abstract]
Treatment of Metastatic Childhood Soft Tissue Sarcoma
Standard treatment options for metastatic childhood soft tissue sarcoma include the following:
- Multimodality therapy using chemotherapy, radiation therapy, and surgical resection of pulmonary metastases.
For treatment options, see the individual tumor type sections of the summary.
The prognosis for children with metastatic soft tissue sarcomas is poor.[1-6] In a prospective randomized trial, chemotherapy with vincristine, dactinomycin, doxorubicin, and cyclophosphamide, with or without dacarbazine, led to tumor responses in one-third of patients with unresectable or metastatic disease. However, the estimated 4-year survival rate was poor. Less than one-third of children survived.[6-8]
Targeted (stereotactic body) radiation therapy is an option for sites of metastasis, particularly the lung.[9] Targeted radiation therapy is also an option for local control or sites of metastasis, including the lungs, bones, and brain,[10,11] particularly in patients for whom the morbidity of resection is a concern or whose life expectancy is limited.[9]
In a prospective trial of children with metastatic soft tissue sarcoma, patients were randomly assigned to receive multiagent chemotherapy with or without the addition of bevacizumab.[12] There was no difference in event-free survival or overall survival between the two study arms.
Pulmonary Metastases
Generally, a surgical procedure, with resection of all gross disease, should be considered for children with isolated pulmonary metastases.[13] For patients with multiple or recurrent pulmonary metastases, additional surgical procedures can be performed if the morbidity is deemed acceptable. In a retrospective review, patients with synovial sarcoma and pulmonary metastases who underwent complete resection of all metastatic lung lesions had better survival than patients who did not undergo complete resections.[13][Level of evidence C1] Formal segmentectomy, lobectomy, and mediastinal lymph node dissection are unnecessary.[14]
An alternative approach is focused radiation therapy (fractionated stereotactic radiation therapy), which has been successfully used in adults to control lesions. The estimated 5-year survival rate after thoracotomy for pulmonary metastasectomy has ranged from 10% to 58% in adult studies.[9]
References
- Demetri GD, Elias AD: Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 9 (4): 765-85, 1995. [PUBMED Abstract]
- Elias A, Ryan L, Sulkes A, et al.: Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol 7 (9): 1208-16, 1989. [PUBMED Abstract]
- Edmonson JH, Ryan LM, Blum RH, et al.: Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 11 (7): 1269-75, 1993. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Dhakal S, Corbin KS, Milano MT, et al.: Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 82 (2): 940-5, 2012. [PUBMED Abstract]
- Howard TP, Boyle PJ, Marcus KJ, et al.: Clinical outcomes for pediatric patients receiving radiotherapy for solid tumor central nervous system metastases. Pediatr Blood Cancer 68 (12): e29331, 2021. [PUBMED Abstract]
- Cameron AL, Elze MC, Casanova M, et al.: The Impact of Radiation Therapy in Children and Adolescents With Metastatic Rhabdomyosarcoma. Int J Radiat Oncol Biol Phys 111 (4): 968-978, 2021. [PUBMED Abstract]
- Ferrari A, Merks JHM, Chisholm JC, et al.: Outcomes of metastatic non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) treated within the BERNIE study: a randomised, phase II study evaluating the addition of bevacizumab to chemotherapy. Eur J Cancer 130: 72-80, 2020. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Putnam JB, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
Treatment of Progressive or Recurrent Childhood Soft Tissue Sarcoma
With the possible exception of infants with infantile fibrosarcoma, the prognosis for patients with progressive or recurrent disease is poor. No prospective trial has demonstrated that enhanced local control of pediatric soft tissue sarcomas will ultimately improve survival. Therefore, treatment should be individualized for the site of recurrence, biological characteristics of the tumor (e.g., grade, invasiveness, and size), previous therapies, and individual patient considerations. All patients with recurrent tumors should consider participating in clinical trials.
Published results of two studies addressed the outcomes of children with relapsed synovial sarcoma. Most patients in one study had distant relapse (29 of 44 patients),[1] while most patients in the second study had local relapse (27 of 37 patients).[2] Distant recurrence was a poor prognostic variable, while tumor resectability at relapse (as manifested by extremity recurrence) was associated with a better outcome in both studies.
Resection is the standard treatment for recurrent pediatric nonrhabdomyosarcomatous soft tissue sarcomas. If the patient has not yet received radiation therapy, postoperative radiation should be considered after local excision of the recurrent tumor. Limb-sparing procedures with postoperative brachytherapy have been evaluated in adults but have not been studied extensively in children. For some children with extremity sarcomas who have received previous radiation therapy, amputation may be the only therapeutic option.
Treatment options for progressive or recurrent disease include the following:
Surgery
Evidence (surgery):
- Surgical excision of local recurrence.
- An Italian review of 73 patients with recurrent malignant peripheral nerve sheath tumors found that most relapses were local.[3][Level of evidence C1]
- Multivariate analysis showed that the factors associated with improved survival were no tumor invasiveness at initial diagnosis (stage T1), time of recurrence more than 12 months after initial diagnosis, and achievement of a second complete response with surgical removal of the recurrence(s).
- Only 15.8% of patients who had complete surgical excisions of local recurrence(s) were alive at 5 years.
- An Italian review of 73 patients with recurrent malignant peripheral nerve sheath tumors found that most relapses were local.[3][Level of evidence C1]
- Surgical excision of isolated pulmonary occurrence.
- Pulmonary metastasectomy may achieve prolonged disease control for some patients.[4]
- A large, retrospective analysis showed that patients with recurrent soft tissue sarcoma and isolated local relapse had a better prognosis and resection of pulmonary metastases improved the probability of survival.[5]
- In 31 children and adolescents younger than 23 years with pulmonary metastases from synovial sarcoma, complete resection of lung metastases appeared to prolong survival when compared with ten other patients who were not candidates for metastasectomy.[6][Level of evidence C1]
- Pulmonary metastasectomy may achieve prolonged disease control for some patients.[4]
- Surgical excision of local recurrence followed by radiation therapy or brachytherapy (if no previous radiation therapy was given).
- Limb amputation (only for some children with extremity sarcomas that have already received radiation therapy).
Chemotherapy
Chemotherapy agents that have been used to treat recurrent soft tissue sarcomas include the following:
Tyrosine Kinase Inhibitors
Evidence (tyrosine kinase inhibitors):
- Pazopanib. The U.S. Food and Drug Administration approved pazopanib for use in patients with recurrent soft tissue sarcoma. The clinical trial that led to the approval was limited to adults. The study demonstrated disease stabilization and prolonged time to progression; it did not demonstrate improved overall survival.[11]
- A phase I trial of pazopanib reported one partial response in a patient with desmoplastic small round cell tumor and prolonged disease stabilization in eight patients with recurrent sarcomas.[12][Level of evidence B4]
- One 13-year-old boy and one 14-year-old girl with multiply recurrent synovial sarcoma and lung metastases had responses to pazopanib for 14 and 15 months, respectively.[13][Level of evidence C2]
Immune Checkpoint Inhibitors
Evidence (immune checkpoint inhibitors):
- Pembrolizumab. The Sarcoma Alliance for Research through Collaboration conducted a phase II trial of the checkpoint inhibitor pembrolizumab in patients aged 18 years and older with recurrent soft tissue sarcoma.[14][Level of evidence C3]
- Seven of 40 patients (18%) with soft tissue sarcoma had objective responses.
- Four of ten patients (40%) with undifferentiated pleomorphic sarcoma, two of ten patients (20%) with liposarcoma, and one of ten patients (10%) with synovial sarcoma had objective responses.
- No patients with leiomyosarcoma (n = 10) had an objective response.
Treatment Options Under Clinical Evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Soole F, Maupain C, Defachelles AS, et al.: Synovial sarcoma relapses in children and adolescents: prognostic factors, treatment, and outcome. Pediatr Blood Cancer 61 (8): 1387-93, 2014. [PUBMED Abstract]
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Belal A, Salah E, Hajjar W, et al.: Pulmonary metastatectomy for soft tissue sarcomas: is it valuable? J Cardiovasc Surg (Torino) 42 (6): 835-40, 2001. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 57 (3): 739-47, 2003. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Le Cesne A, Cresta S, Maki RG, et al.: A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer 48 (16): 3036-44, 2012. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Maki RG, et al.: Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 23 (24): 5484-92, 2005. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Manola J, et al.: Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 22 (8): 1480-90, 2004. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Glade Bender JL, Lee A, Reid JM, et al.: Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol 31 (24): 3034-43, 2013. [PUBMED Abstract]
- Casanova M, Basso E, Magni C, et al.: Response to pazopanib in two pediatric patients with pretreated relapsing synovial sarcoma. Tumori 103 (1): e1-e3, 2017. [PUBMED Abstract]
- Tawbi HA, Burgess M, Bolejack V, et al.: Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 18 (11): 1493-1501, 2017. [PUBMED Abstract]
Latest Updates to This Summary (04/21/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Childhood Soft Tissue Sarcoma
Revised Table 2 to include ROS1 as a gene involved in inflammatory myofibroblastic tumor (cited Mariño-Enríquez et al. and Lovly et al. as references 70 and 71, respectively).
Treatment Option Overview for Childhood Soft Tissue Sarcoma
Revised text to state that in the Children's Oncology Group (COG) ARST0332 study, if the central review surgeon deemed the tumor unresectable without loss of limb, form, or function, the patient was treated in arm C with neoadjuvant radiation therapy. Surgery was performed 4 to 6 weeks after the completion of radiation therapy. This early surgery allowed for decreased morbidity, better wound healing, and more complete surgical resection. Also revised text to state that while there should be an emphasis on achieving negative margins without loss of form or function, given the variability of chemosensitivity of such diverse tumors, it may be better to tailor the resection to histology for each patient.
Treatment of Fibroblastic and Myofibroblastic Tumors
Added text about the results of a COG trial of larotrectinib in 33 children with NTRK fusion–positive solid tumors, including 18 patients with infantile fibrosarcoma (cited Laetsch et al. as reference 100).
Added text to state that a study by the Ultra-Rare Sarcoma Working Group examined 32 patients with low-grade fibromyxoid sarcoma and rare occurrences of distant metastases. Most metastases occurred in the lungs. Treatments varied, and minimal responses were observed to anthracycline-based and gemcitabine-based regimens with trabectidin. However, there were few patients in each treatment group (cited Giani et al. as reference 111).
Treatment of Tumors of Uncertain Differentiation
Added text about the results of an open-label, international, phase II study that enrolled patients with previously treated metastatic or unresectable synovial sarcoma and myxoid round cell liposarcoma. Fifty-two patients with HLA-A*02 and tumors that expressed melanoma-associated antigen A4 (MAGE-A4) received a single intravenous dose of afamitresgene autoleucel (afami-cel) after lymphodepletion (cited D'Angelo et al. as reference 53). Also added text to state that the data from this trial led to U.S. Food and Drug Administration approval of afami-cel in adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA-A*02 positive, and whose tumors express the MAGE-A4 antigen.
Added atezolizumab as a targeted therapy that has been studied in patients with advanced alveolar soft part sarcoma. Also added text about the results of a phase II trial of 52 patients older than 2 years who were treated with atezolizumab (cited Chen et al. as reference 82).
Added Intracranial Mesenchymal Tumor as a new subsection.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood soft tissue sarcoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Soft Tissue Sarcoma Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Holcombe Edwin Grier, MD
- Andrea A. Hayes-Dixon, MD, FACS, FAAP (Howard University)
- William H. Meyer, MD
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/child-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389361]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.