Oropharyngeal Cancer Treatment (PDQ®)–Health Professional Version
General Information About Oropharyngeal Cancer
Incidence and Mortality
Estimated new cases and deaths from cancer of the oral cavity and pharynx in the United States in 2025:[1]
- New cases: 59,660.
- Deaths: 12,770.
The increasing incidence of oropharyngeal cancer is attributed to the rise in human papillomavirus (HPV)-associated cases. Men are almost three times as likely as women to have oropharyngeal cancer.[1-3]
Anatomy
Anatomically, the oropharynx is located between the soft palate superiorly and the hyoid bone inferiorly. It is continuous with the oral cavity anteriorly and communicates with the nasopharynx superiorly and the supraglottic larynx and hypopharynx inferiorly.
The oropharynx is divided into the following parts:[4]
- Base of the tongue, which includes the pharyngoepiglottic folds and the glossoepiglottic folds.
- Vallecula.
- Tonsillar region, which includes the fossa and the anterior and posterior pillars.
- Soft palate, which includes the uvula.
- Posterior and lateral pharyngeal walls.
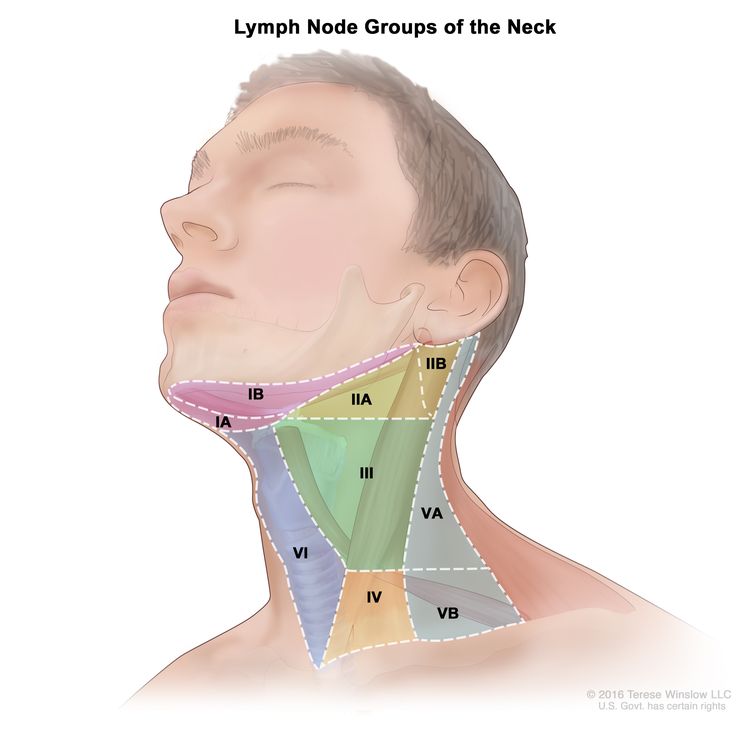
Regional lymph node anatomy of the head and neck
The regional lymph nodes of the head and neck include the lymph nodes that run parallel to the jugular veins, spinal accessory nerve, and facial artery and into the submandibular triangle. An understanding of regional anatomy and the status of regional lymph nodes is critical to the care of patients with head and neck cancer.[3,5,6] To facilitate communication regarding lymph node anatomy, the regions of the neck are described as levels I to V and retropharyngeal:
- Level I contains the submental and submandibular lymph nodes.
- Level II contains the upper jugular lymph nodes, which are above the digastric muscle.
- Level III contains the midjugular lymph nodes, which are between the omohyoid muscle and the digastric muscle.
- Level IV contains the lower jugular lymph nodes.
- Level V contains the lymph nodes of the posterior triangle.
- Retropharyngeal lymph nodes.
The retropharyngeal lymph nodes are a possible site for nodal spread in oropharyngeal cancer. A large retrospective cohort study from the MD Anderson Cancer Center described the clinical features of 981 patients with oropharyngeal cancer who underwent primary radiation therapy.[7][Level of evidence C1][Level of evidence C2]
- The base of the tongue (47%) and the tonsil (46%) were the most common primary sites.
- Most patients had stage T1 to T2 primary tumors (64%) and stage III to IVB disease (94%).
- The incidence of radiographic retropharyngeal node involvement was 10% and was highest for the pharyngeal wall (23%) and lowest for the base of the tongue (6%).
- Retropharyngeal lymph node involvement was associated with inferior 5-year local control and inferior recurrence-free survival, distant metastases−free survival, and overall survival on multivariate analysis.
Risk Factors
Risk factors for oropharyngeal squamous cell carcinoma (SCC) include:[8]
- Smoking history of more than 10 pack years and other tobacco use.[9,10]
- Heavy alcohol use.
- HPV infection, especially HPV type 16, also known as HPV-16.[11-13]
- Personal history of head and neck cancer.
- Betel quid chewing.
For more information, see Oral Cavity, Oropharyngeal, Hypopharyngeal, and Laryngeal Cancers Prevention.
HPV infection
Because of the decreased incidence of smoking in the United States, HPV-negative, smoking-related oropharyngeal cancer is decreasing; however, HPV-positive oropharyngeal cancer is increasing. According to the Surveillance, Epidemiology, and End Results (SEER) Program's tissue repository data from 1988 to 2004, the prevalence of HPV-negative oropharyngeal cancer declined by 50%, while HPV-positive oropharyngeal cancers increased by 225%.[14][Level of evidence C1]
HPV-positive oropharyngeal cancers may represent a distinct disease entity that is caused by HPV infection and associated with an improved prognosis. Several studies indicate that individuals with HPV-positive tumors have significantly improved survival.[12,15-17] Due to the prognostic impact of the HPV status in oropharyngeal cancer, the American Joint Committee on Cancer 8th edition staging separates oropharyngeal staging by HPV status.[5,6] In a prospective study of 253 patients with newly diagnosed or recurrent head and neck SCC, HPV was detected in 25% of the patients. Poor tumor grade and an oropharyngeal site independently increased the probability of the presence of HPV.[12] Oropharyngeal tumors are more likely to be HPV positive (57%) than oral cavity (12%) tumor sites and non-oropharyngeal (14%) sites. HPV-positive oropharyngeal cancers predominantly arise in the palatine or lingual tonsils. For tonsil or base-of-tongue sites, 62% of tumors were HPV positive, compared with 25% for other oropharyngeal sites.
Personal history of head and neck cancer
The risk of developing a second primary tumor in patients with tumors of the upper aerodigestive tract has been estimated to be 3% to 7% per year.[18,19] Because of this risk, patients require lifelong surveillance. Smoking and alcohol consumption after treatment are associated with the development of second primary tumors of the aerodigestive tract.[20-22] Patients may need counseling to discontinue smoking and alcohol consumption.
The process of field cancerization may be partly responsible for the multiple, synchronous, primary SCCs that occur in oropharyngeal cancer and that are associated with a smoking history. Originally described in 1953, the concept of field cancerization holds that tumors develop in a multifocal fashion within a field of tissue chronically exposed to carcinogens.[23] Molecular studies that detect genetic alterations in histologically normal tissue from high-risk individuals have provided strong support for this concept.[24-28]
A comparison of patients (N = 2,230) with index SCC of the oropharynx site and index SCC of non-oropharyngeal sites (i.e., oral cavity, larynx, and hypopharynx) was performed to determine the likelihood of developing second primary malignancies. The second primary malignancy rate was lower for patients with index oropharyngeal SCC than for patients with index non-oropharyngeal cancer (P < .001). Among patients with oropharyngeal SCC, former smokers had a 50% higher risk of second primary malignancy than never-smokers, and current smokers had a 100% higher risk than never-smokers (P trend = .008). These data suggest that patients who fit the typical HPV phenotype have a very low risk of second primary malignancy.[29]
Betel quid
The chewing of betel quid, a stimulant preparation commonly used in parts of Asia, increases the risk of oropharyngeal cancer.[30]
Other risk factors
Other risk factors may include:[8]
- Defective elimination of acetaldehyde, a carcinogen generated by alcohol metabolism. In individuals, primarily those of East Asian race, who carry an inactive mutant allele of alcohol dehydrogenase-2, alcohol consumption is associated with a susceptibility to multiple metachronous oropharyngeal cancers that are caused by the decreased elimination of acetaldehyde.[31]
SCC of the oropharynx has not been associated with any specific chromosomal or genetic abnormalities. Genetic and chromosomal aberrations in these cancers are complex.[32,33] Despite the lack of specific genetic abnormalities, testing for genetic alterations or ploidy in early oropharyngeal lesions may identify patients who are at the greatest risk of disease progression and may lead to more-definitive therapy.[34]
Clinical Presentation
The clinical presentation of oropharyngeal cancer depends on the tumor’s location in the oropharynx. Oropharyngeal cancer may present in the following locations:
- Tonsil, tonsillar fossa, tonsillar pillars, or glossotonsillar sulci.
- Base of the tongue (posterior one-third of the tongue posterior to the circumvallate papillae).
- Vallecula.
- Soft palate, inferior surface, or uvula.
- Posterior pharyngeal wall.
Tonsil
The anterior tonsillar pillar and tonsil are the most common location for a primary tumor of the oropharynx.[4] Lesions involving the anterior tonsillar pillar may appear as areas of dysplasia, inflammation, or a superficial spreading lesion. These cancers can spread across a broad region, including the lateral soft palate, retromolar trigone and buccal mucosa, and tonsillar fossa.[3,4] The lymphatic drainage is primarily to level II nodes.
Tumors of the posterior tonsillar pillar can extend inferiorly to involve the pharyngoepiglottic fold and the posterior aspect of the thyroid cartilage. These lesions more frequently involve level V nodes.
Lesions of the tonsillar fossa may be either exophytic or ulcerative and have a pattern of extension similar to those of the anterior tonsillar pillar. These tumors present as advanced-stage disease more often than do cancers of the tonsillar pillar. Approximately 75% of patients will present with stage III or stage IV disease.[3,4] The lymphatic drainage is primarily to level V nodes. Tumors of the posterior tonsillar pillar can extend inferiorly to involve the pharyngoepiglottic fold and the posterior aspect of the thyroid cartilage. These lesions more frequently involve level V nodes.
Signs and symptoms of tonsillar lesions may include:[3,4]
- Pain.
- Dysphagia.
- Weight loss.
- Ipsilateral referred otalgia.
- A mass in the neck.
Base of the tongue
Clinically, cancers of the base of the tongue are insidious. These cancers can grow in either an infiltrative or exophytic pattern. Because the base of the tongue is devoid of pain fibers, these tumors are often asymptomatic until there is significant tumor progression.[4]
Signs and symptoms of advanced base-of-the-tongue cancers may include:[3,4]
- Pain.
- Dysphagia.
- Weight loss.
- Referred otalgia secondary to cranial nerve involvement.
- Trismus secondary to pterygoid muscle involvement.
- Fixation of the tongue that is caused by infiltration of the deep muscle.
- A mass in the neck.
Lymph node metastasis is common because of the rich lymphatic drainage of the base of the tongue. Approximately 70% or more of patients with advanced base-of-the-tongue cancers have ipsilateral cervical nodal metastases; 30% or fewer of such patients have bilateral, cervical lymph–node metastases.[4,35] The cervical lymph nodes involved commonly include levels II, III, IV, and V and retropharyngeal lymph nodes.
Soft palate
Soft palate tumors are primarily found on the anterior surface.[4] Lesions in this area may remain superficial and in early stages.[3] The lymphatic drainage is primarily to level II nodes.
Pharyngeal wall
Pharyngeal wall lesions can spread superiorly to involve the nasopharynx, posteriorly to infiltrate the prevertebral fascia, and inferiorly to involve the pyriform sinuses and hypopharyngeal walls. Primary lymphatic drainage is to the retropharyngeal nodes and level II and III nodes. Because most pharyngeal tumors extend past the midline, bilateral cervical metastases are common.
Early-stage tumors are often asymptomatic. Tumors of the pharyngeal wall are typically diagnosed in an advanced stage.[3,4]
Signs and symptoms of advanced pharyngeal wall tumors may include:
- Pain.
- Bleeding.
- Dysphagia.
- Weight loss.
- A mass in the neck.
Leukoplakia
Leukoplakia is used only as a clinically descriptive term meaning that the observer sees a white patch that does not rub off, the significance of which depends on the histological findings.[8] Leukoplakia can range from hyperkeratosis to an actual early invasive carcinoma or may represent a fungal infection, lichen planus, or other benign oral disease.
Diagnostic Evaluation
The assessment of the primary tumor is based on inspection and palpation, when possible, and by indirect mirror examination. The appropriate nodal drainage areas are examined by careful palpation. The presence of tumor must be confirmed histologically. Any other pathological data obtained from a biopsy and additional radiographical studies are also considered.
The following procedures may be done to evaluate the primary tumor:
- Positron emission tomography–computed tomography (PET-CT) scan.
- Magnetic resonance imaging.
- Endoscopy.
- Laryngoscopy.
- Biopsy and p16 testing to assess for HPV status.
A PET-CT scan yields morphological and metabolic data to assess the detection of primary tumor, nodal disease, and distant metastatic disease. It may also be used to guide radiation therapy planning. Retrospective data demonstrate that morphological and PET-glycolytic parameters (as measured by fluorodeoxyglucose PET-CT) are significantly larger (as measured by Response Evaluation Criteria In Solid Tumors [RECIST] longest diameter) and more heterogenous in HPV-negative disease than in HPV-positive disease in the primary tumor for oropharyngeal carcinoma. These PET-CT parameters also show higher standardized uptake value (SUV) max, SUV mean, and metabolic tumor volume in HPV-negative disease. However, the same PET parameters are frequently larger in the regional nodal disease in patients with HPV-positive disease.[36][Level of evidence C3]
Prognostic Factors and Survival
Prognostic factors for oropharyngeal carcinoma include:
- HPV status.
- Smoking history (10 or more pack-years).
- Tumor stage and nodal status.
The criteria described in Table 1 are used to determine whether patients have low-, intermediate-, or high-risk oropharyngeal carcinoma. These criteria have been defined by using recursive partitioning analysis in a retrospective analysis of a randomized trial of patients with stage III and IV oropharyngeal SCC treated with chemoradiation.[17]
| Degree of Risk | Characteristics | 3-y OS Rate |
|---|---|---|
| CI = confidence interval; HPV = human papillomavirus; OS = overall survival; + = positive; - = negative. For more information, see the AJCC Staging Groupings and TNM Definitions section. | ||
| aAng KK, Harris J, Wheeler R, et al.: Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363 (1): 24–35, 2010. | ||
| Low | HPV+, smoking history of ≤10 pack-years, and N0–N2a nodal history | 93% (95% CI, 88.3%–97.7%) |
| Intermediate | HPV+, smoking history of >10 pack-years, and N2b–N3 nodal disease; or | 70.8% (95% CI, 60.7%–80.8%) |
| HPV-, smoking history of ≤10 pack-years, and N2b–N3 nodal disease or T2–T3 tumors | ||
| High | HPV- and smoking history >10 pack-years; or | 46.2% (95% CI, 34.7%–57.7%) |
| HPV-, smoking history ≤10 pack-years, and T4 disease | ||
Follow-Up After Treatment
A careful examination of the patient's head and neck allows the physician to look for recurrence every 6 to 12 weeks for the first posttreatment year, every 3 months for the second year, every 3 to 4 months for the third year, and every 6 months thereafter.
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Bray F, Laversanne M, Sung H, et al.: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74 (3): 229-263, 2024. [PUBMED Abstract]
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Choi WH, Hu KS, Culliney B, et al.: Cancer of the oropharynx. In: Harrison LB, Sessions RB, Hong WK, eds.: Head and Neck Cancer: A Multidisciplinary Approach. 3rd ed. Lippincott, William & Wilkins, 2009, pp 285-335.
- HPV-Mediated (p16+) Oropharyngeal Cancer. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 113-21.
- Oropharynx (p16-) and Hypopharynx. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 123-35.
- Gunn GB, Debnam JM, Fuller CD, et al.: The impact of radiographic retropharyngeal adenopathy in oropharyngeal cancer. Cancer 119 (17): 3162-9, 2013. [PUBMED Abstract]
- Neville BW, Day TA: Oral cancer and precancerous lesions. CA Cancer J Clin 52 (4): 195-215, 2002 Jul-Aug. [PUBMED Abstract]
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Licitra L, Bernier J, Grandi C, et al.: Cancer of the oropharynx. Crit Rev Oncol Hematol 41 (1): 107-22, 2002. [PUBMED Abstract]
- Mork J, Lie AK, Glattre E, et al.: Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 344 (15): 1125-31, 2001. [PUBMED Abstract]
- Gillison ML, Koch WM, Capone RB, et al.: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92 (9): 709-20, 2000. [PUBMED Abstract]
- D'Souza G, Kreimer AR, Viscidi R, et al.: Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356 (19): 1944-56, 2007. [PUBMED Abstract]
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al.: Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29 (32): 4294-301, 2011. [PUBMED Abstract]
- Ringström E, Peters E, Hasegawa M, et al.: Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res 8 (10): 3187-92, 2002. [PUBMED Abstract]
- Schwartz SR, Yueh B, McDougall JK, et al.: Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg 125 (1): 1-9, 2001. [PUBMED Abstract]
- Ang KK, Harris J, Wheeler R, et al.: Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363 (1): 24-35, 2010. [PUBMED Abstract]
- Khuri FR, Lippman SM, Spitz MR, et al.: Molecular epidemiology and retinoid chemoprevention of head and neck cancer. J Natl Cancer Inst 89 (3): 199-211, 1997. [PUBMED Abstract]
- León X, Quer M, Diez S, et al.: Second neoplasm in patients with head and neck cancer. Head Neck 21 (3): 204-10, 1999. [PUBMED Abstract]
- Do KA, Johnson MM, Doherty DA, et al.: Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control 14 (2): 131-8, 2003. [PUBMED Abstract]
- Khuri FR, Kim ES, Lee JJ, et al.: The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev 10 (8): 823-9, 2001. [PUBMED Abstract]
- Day GL, Blot WJ, Shore RE, et al.: Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst 86 (2): 131-7, 1994. [PUBMED Abstract]
- Slaughter DP, Southwick HW, Smejkal W: Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer 6 (5): 963-8, 1953. [PUBMED Abstract]
- Braakhuis BJ, Tabor MP, Leemans CR, et al.: Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck 24 (2): 198-206, 2002. [PUBMED Abstract]
- Braakhuis BJ, Tabor MP, Kummer JA, et al.: A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res 63 (8): 1727-30, 2003. [PUBMED Abstract]
- Tabor MP, Brakenhoff RH, van Houten VM, et al.: Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res 7 (6): 1523-32, 2001. [PUBMED Abstract]
- Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, et al.: Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am J Pathol 161 (3): 1051-60, 2002. [PUBMED Abstract]
- Ha PK, Califano JA: The molecular biology of mucosal field cancerization of the head and neck. Crit Rev Oral Biol Med 14 (5): 363-9, 2003. [PUBMED Abstract]
- Gan SJ, Dahlstrom KR, Peck BW, et al.: Incidence and pattern of second primary malignancies in patients with index oropharyngeal cancers versus index nonoropharyngeal head and neck cancers. Cancer 119 (14): 2593-601, 2013. [PUBMED Abstract]
- Ho PS, Ko YC, Yang YH, et al.: The incidence of oropharyngeal cancer in Taiwan: an endemic betel quid chewing area. J Oral Pathol Med 31 (4): 213-9, 2002. [PUBMED Abstract]
- Yokoyama A, Watanabe H, Fukuda H, et al.: Multiple cancers associated with esophageal and oropharyngolaryngeal squamous cell carcinoma and the aldehyde dehydrogenase-2 genotype in male Japanese drinkers. Cancer Epidemiol Biomarkers Prev 11 (9): 895-900, 2002. [PUBMED Abstract]
- Tremmel SC, Götte K, Popp S, et al.: Intratumoral genomic heterogeneity in advanced head and neck cancer detected by comparative genomic hybridization. Cancer Genet Cytogenet 144 (2): 165-74, 2003. [PUBMED Abstract]
- Brieger J, Jacob R, Riazimand HS, et al.: Chromosomal aberrations in premalignant and malignant squamous epithelium. Cancer Genet Cytogenet 144 (2): 148-55, 2003. [PUBMED Abstract]
- Forastiere A, Koch W, Trotti A, et al.: Head and neck cancer. N Engl J Med 345 (26): 1890-900, 2001. [PUBMED Abstract]
- Lindberg R: Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 29 (6): 1446-9, 1972. [PUBMED Abstract]
- Tahari AK, Alluri KC, Quon H, et al.: FDG PET/CT imaging of oropharyngeal squamous cell carcinoma: characteristics of human papillomavirus-positive and -negative tumors. Clin Nucl Med 39 (3): 225-31, 2014. [PUBMED Abstract]
Cellular Classification of Oropharyngeal Cancer
Most oropharyngeal cancers are squamous cell carcinomas (SCCs).[1-3] SCCs may be noninvasive or invasive. For noninvasive SCC, the term carcinoma in situ is used. Histologically, invasive carcinomas are classified as well differentiated, moderately differentiated, poorly differentiated, or undifferentiated. SCCs are usually moderately or poorly differentiated.[4] Grading the deep invasive margins (i.e., invasive front) of SCC may provide better prognostic information than grading the entire tumor.[5] Human papillomavirus (HPV)-positive oropharyngeal cancers arising from the lingual and palatine tonsils are a distinct molecular-pathological entity that is linked to infection with HPV, especially HPV-16. Compared with HPV-negative tumors, HPV-positive tumors are more frequently poorly differentiated and nonkeratinizing. They are strongly associated with basaloid morphology and less likely to have TP53 variants.[6]
Immunohistochemical examination of tissues for the expression of the biomarker Ki-67, a proliferation antigen, may complement histological grading. As a molecular indicator of epithelial dysplasia of the oropharynx, Ki-67 expression appears to correlate well with loss of heterozygosity (LOH) in tumor cells. In a retrospective study involving 43 tissue samples from 25 patients, the assessment of proliferation with Ki-67 was a better surrogate for LOH than was histological grading.[7]
Other types of oropharyngeal cancer include:
- Minor salivary gland tumors.
- Lymphomas.
- Lymphoepitheliomas (e.g., tonsillar fossa).
For more information, see Salivary Gland Cancer Treatment, Hodgkin Lymphoma Treatment, Indolent B-Cell Non-Hodgkin Lymphoma Treatment, Aggressive B-Cell Non-Hodgkin Lymphoma Treatment, and Peripheral T-Cell Non-Hodgkin Lymphoma Treatment.
References
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- HPV-Mediated (p16+) Oropharyngeal Cancer. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 113-21.
- Oropharynx (p16-) and Hypopharynx. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 123-35.
- Oral cavity and oropharynx. In: Rosai J, ed.: Rosai and Ackerman's Surgical Pathology. Vol. 1. 10th ed. Mosby Elsevier, 2011, pp. 237-264.
- Bryne M, Boysen M, Alfsen CG, et al.: The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer Res 18 (6B): 4757-64, 1998 Nov-Dec. [PUBMED Abstract]
- Gillison ML, Koch WM, Capone RB, et al.: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92 (9): 709-20, 2000. [PUBMED Abstract]
- Tabor MP, Braakhuis BJ, van der Wal JE, et al.: Comparative molecular and histological grading of epithelial dysplasia of the oral cavity and the oropharynx. J Pathol 199 (3): 354-60, 2003. [PUBMED Abstract]
Stage Information for Oropharyngeal Cancer
The staging system for oropharyngeal cancer is clinical rather than pathological. It is based on the best estimate of the extent of disease before treatment.
Clinical anatomical staging of oropharyngeal cancer involves the following clinical assessment and imaging techniques:
- Inspection and palpation of sites (when feasible) and neck nodes.
- Neurological examination of all cranial nerves.
- A head and neck computed tomography (CT) scan with contrast to evaluate the mandible and maxilla.[1]
- Magnetic resonance imaging to evaluate the extent of disease in the soft tissues.
- Positron emission tomography (PET)–CT scan to assess primary, regional, and distant metastatic spread.
- Complete endoscopy after completion of other staging studies to assess the surface extent of the tumor accurately and to facilitate biopsy. This procedure is typically performed under general anesthesia, which also allows palpation for deep muscle invasion. Because of the incidence of multiple primary tumors occurring simultaneously, a careful search for other primary tumors of the upper aerodigestive tract is indicated.[2]
PET has been investigated as an imaging modality for recurrent oropharyngeal cancer.[3]
American Joint Committee on Cancer (AJCC) Staging Groupings and TNM Definitions
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define oropharyngeal cancer.[2,4] Nonepithelial tumors such as those of lymphoid tissue, soft tissue, bone, and cartilage are not included.
The AJCC uses separate staging systems for human papillomavirus (HPV)-related squamous cell carcinoma of the oropharynx [4] and p16-negative squamous cancers of the oropharynx.[2]
AJCC prognostic stage groups for HPV-mediated (p16-positive) oropharyngeal cancer
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: HPV-Mediated (p16+) Oropharyngeal Cancer. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 113–21. | ||
| I | T0, T1, or T2; N0 or N1; M0 | T0, T1, or T2 = See Stage IV in Table 5 below. |
| N0 or N1 = See Stage IV in Table 5 below. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: HPV-Mediated (p16+) Oropharyngeal Cancer. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 113–21. | ||
| II | T0, T1, or T2; N2; M0 | T0, T1, or T2 = See Stage IV in Table 5 below. |
| N2 = Contralateral or bilateral lymph nodes, none >6 cm. | ||
| M0 = No distant metastasis. | ||
| T3; N0, N1, or N2; M0 | T3 = Tumor >4 cm in greatest dimension or extension to lingual surface of the epiglottis. | |
| N0, N1, or N2 = See Stage IV in Table 5 below. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: HPV-Mediated (p16+) Oropharyngeal Cancer. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 113–21. | ||
| bMucosal extension to the lingual surface of the epiglottis from primary tumors of the base of the tongue and vallecula does not constitute invasion of the larynx. | ||
| III | T0, T1, T2, T3, or T4; N3; M0 | T0, T1, T2, T3, or T4 = See Stage IV in Table 5 below. |
| N3 = Lymph node(s) >6 cm. | ||
| M0 = No distant metastasis. | ||
| T4; N0, N1, N2, or N3; M0 | T4 = Moderately advanced local disease. Tumor invades the larynx, extrinsic muscle of the tongue, medial pterygoid, hard palate, or mandible or beyond.b | |
| N0, N1, N2, or N3 = See Stage IV in Table 5 below. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: HPV-Mediated (p16+) Oropharyngeal Cancer. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 113–21. | ||
| bMucosal extension to the lingual surface of the epiglottis from primary tumors of the base of the tongue and vallecula does not constitute invasion of the larynx. | ||
| IV | Any T, Any N, M1 | T0 = No primary identified. |
| T1 = Tumor ≤2 cm in greatest dimension. | ||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension. | ||
| T3 = Tumor >4 cm in greatest dimension or extension to lingual surface of the epiglottis. | ||
| T4 = Moderately advanced local disease. Tumor invades the larynx, extrinsic muscle of the tongue, medial pterygoid, hard palate, or mandible or beyond.b | ||
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node metastasis. | ||
| N1 = One or more ipsilateral lymph nodes, none >6 cm. | ||
| N2 = Contralateral or bilateral lymph nodes, none >6 cm. | ||
| N3 = Lymph node(s) >6 cm. | ||
| M1 = Distant metastasis. | ||
AJCC prognostic stage groups for p16-negative squamous cancers of the oropharynx
| Stage | TNbM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Oropharynx (p16−) and Hypopharynx. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 123–35. | ||
| The explanation for superscript b is at the end of Table 10. | ||
| 0 | Tis, N0, M0 | Tis = Carcinoma in situ. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNbM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Oropharynx (p16−) and Hypopharynx. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 123–35. | ||
| The explanation for superscript b is at the end of Table 10. | ||
| I | T1, N0, M0 | T1 = Tumor ≤2 cm in greatest dimension. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNbM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Oropharynx (p16−) and Hypopharynx. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 123–35. | ||
| The explanation for superscript b is at the end of Table 10. | ||
| II | T2, N0, M0 | T2 = Tumor >2 cm but ≤4 cm in greatest dimension. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNbM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; ENE = extranodal extension. | ||
| aReprinted with permission from AJCC: Oropharynx (p16−) and Hypopharynx. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 123–35. | ||
| The explanation for superscript b is at the end of Table 10. | ||
| III | T3, N0, M0 | T3 = Tumor >4 cm in greatest dimension or extension to lingual surface of epiglottis. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| T1, T2, T3; N1; M0 | T1, T2, T3 = See Stage IVC in Table 10 below. | |
| N1 = Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension and ENE(–). | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; ENE = extranodal extension. | ||
| aReprinted with permission from AJCC: Oropharynx (p16−) and Hypopharynx. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 123–35. | ||
| bA designation of U or L may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical and pathological ENE should be recorded as ENE(−) or ENE(+). | ||
| cMucosal extension to the lingual surface of the epiglottis from primary tumors of the base of the tongue and vallecula does not constitute invasion of the larynx. | ||
| IVA | T4a; N0, N1; M0 | T4a = Moderately advanced local disease. Tumor invades the larynx, extrinsic muscle of the tongue, medial pterygoid, hard palate, or mandible.c |
| N0, N1 = See Stage IVC below in this table. | ||
| M0 = No distant metastasis. | ||
| T1, T2, T3, T4a; N2; M0 | T1, T2, T3, T4a = See Stage IVC below in this table. | |
| N2 = Metastasis in a single ipsilateral node >3 cm but ≤6 cm in greatest dimension and ENE(−); or metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE(−); or in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension and ENE(−). | ||
| M0 = No distant metastasis. | ||
| IVB | Any T, N3, M0 | Any T = See Stage IVC below in this table. |
| N3 = Metastasis in a lymph node >6 cm in greatest dimension and ENE(−); or metastasis in any node(s) and clinically overt ENE(+). | ||
| M0 = No distant metastases. | ||
| T4b, Any N, M0 | T4b = Very advanced local disease. Tumor invades lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, or skull base or encases carotid artery. | |
| Any N = See Stage IVC below in this table. | ||
| M0 = No distant metastasis. | ||
| IVC | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. |
| Tis = Carcinoma in situ. | ||
| T1 = Tumor ≤2 cm in greatest dimension. | ||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension. | ||
| T3 = Tumor >4 cm in greatest dimension or extension to lingual surface of epiglottis. | ||
| T4 = Moderately advanced or very advanced local disease. | ||
| −T4a = Moderately advanced local disease. Tumor invades the larynx, extrinsic muscle of the tongue, medial pterygoid, hard palate, or mandible.c | ||
| −T4b = Very advanced local disease. Tumor invades lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, or skull base or encases carotid artery. | ||
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node metastasis. | ||
| N1 = Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension and ENE(−). | ||
| N2 = Metastasis in a single ipsilateral node >3 cm but ≤6 cm in greatest dimension and ENE(−); or metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE(−); or in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension and ENE(−). | ||
| −N2a = Metastasis in a single ipsilateral node >3 cm but ≤6 cm in greatest dimension and ENE(−). | ||
| −N2b = Metastases in multiple ipsilateral nodes, none >6 cm in greatest dimension and ENE(−). | ||
| −N2c = Metastases in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension and ENE(−). | ||
| N3 = Metastasis in a lymph node >6 cm in greatest dimension and ENE(−); or metastasis in any node(s) and clinically overt ENE(+). | ||
| −N3a = Metastasis in a lymph node >6 cm in greatest dimension and ENE(−). | ||
| −N3b = Metastasis in any node(s) and clinically overt ENE(+). | ||
| M1 = Distant metastasis. | ||
References
- Weber AL, Romo L, Hashmi S: Malignant tumors of the oral cavity and oropharynx: clinical, pathologic, and radiologic evaluation. Neuroimaging Clin N Am 13 (3): 443-64, 2003. [PUBMED Abstract]
- Oropharynx (p16-) and Hypopharynx. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 123-35.
- Wong RJ, Lin DT, Schöder H, et al.: Diagnostic and prognostic value of [(18)F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol 20 (20): 4199-208, 2002. [PUBMED Abstract]
- HPV-Mediated (p16+) Oropharyngeal Cancer. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 113-21.
Treatment Option Overview for Oropharyngeal Cancer
An optimal approach for the treatment of oropharyngeal cancer is not easily defined because no single regimen offers a distinct superior-survival advantage. The literature reports various therapeutic options but does not present any valid comparative studies of these options. Treatment considerations should account for functional and performance status, including speech and swallowing outcomes.
Surgery and/or Radiation Therapy
Surgery and radiation therapy have been the standard treatment for oropharyngeal cancer; however, outcome data from randomized trials are limited. Studies have evaluated whether to use surgery or radiation but have been underpowered.[1]
Evidence (surgery and/or radiation therapy):
- In a prospective randomized trial, 564 patients with head and neck cancer and N2 or N3 disease were assigned to either planned neck dissection or surveillance with positron emission tomography–computed tomography (PET-CT) scan.[2]
- With a median follow up of 36 months, PET-CT surveillance resulted in fewer neck dissections compared with the surgical group (54 vs. 221), with a 2-year survival rate of 84.9% for the neck dissection group and 81.5% for the surgical group. The hazard ratio (HR) for death (HRdeath) slightly favored PET-CT−guided surveillance and indicated noninferiority (upper boundary 95% confidence interval [CI] for HR <1.50; P = .004).
- A pooled analysis of 6,400 patients with base-of-the-tongue oropharyngeal carcinoma from 51 reported series between 1970 and 2000 demonstrated the following:[3]
- Local control rates of 79% (surgery with or without radiation therapy) and 76% (radiation therapy alone), (P = .087); locoregional control was 60% for surgery with or without radiation therapy versus 69% for radiation therapy alone (P = .009).
- The 5-year survival rate was 49% for surgery with or without radiation therapy versus 52% (P = .2) for radiation therapy with or without neck dissection.
- The rate of severe complications was 32% for the surgery group versus 3.8% for the radiation therapy group (P < .001).
- The rate of fatal complications was 3.5% for the surgery group versus 0.4% for the radiation therapy group (P < .001).
Historically, the posttreatment performance and functional status of patients with base-of-the-tongue primary tumors was better after radiation therapy than after surgery. Local control and survival are similar in both treatment options.[4,5]
- In the same study, the results for patients with squamous cell carcinoma (SCC) in the tonsillar region who underwent surgery with or without radiation therapy versus radiation therapy with or without neck dissection were as follows:[3]
- Local control rates of 70% (surgery with or without radiation therapy) and 68% (radiation therapy), (P = .2); locoregional control was 65% for surgery with or without radiation therapy versus 69% for radiation therapy alone (P = .1).
- The 5-year survival rate was 47% for surgery with or without radiation therapy versus 43% (P = .2) for radiation therapy with or without neck dissection.
- The rate of severe complications was 23% for the surgery group versus 6% for the radiation therapy group (P < .001)
- The rate of fatal complications was 3.2% for the surgery group versus 0.8% for the radiation therapy group (P < .001).
For patients with early-stage disease, single-modality treatment is preferred. Historically, radiation alone has been preferred, although use of new surgical techniques, including transoral surgery and transoral robotic surgery, is increasing. Nonrandomized comparisons of transoral surgery versus primary radiation therapy suggest superior quality of life (QOL) with minimally invasive surgical techniques.[6] Historically, more–invasive surgical techniques were associated with inferior QOL and greater morbidity.
A prospective multicenter trial (ECOG-3311 [NCT01898494]) evaluating transoral surgery approaches in human papillomavirus−positive oropharyngeal carcinoma with postoperative radiation dose de-escalation is currently under way.
Surgery Followed by Postoperative Radiation Therapy (PORT) With or Without Concurrent Chemotherapy for Patients With Locally Advanced Disease
New surgical techniques for resection and reconstruction that provide access and functional preservation have extended the surgical options for patients with stage III or stage IV oropharyngeal cancer. Specific surgical procedures and their modifications are not described here because of the wide variety of surgical approaches, the variety of opinions about the role of modified neck dissections, and the multiple reconstructive techniques that may give the same results. This group of patients are managed by head and neck surgeons who are skilled in the multiple procedures available and are actively involved in the care of these patients.
Depending on pathological findings after primary surgery, PORT with or without chemotherapy is used in the adjuvant setting for patients with the following histological findings:
- T4 disease.
- Perineural invasion.
- Lymphovascular invasion.
- Positive margins or margins less than 5 mm.
- Extracapsular extension of a lymph node.
- Two or more involved lymph nodes.
The addition of chemotherapy to PORT for oropharyngeal SCC demonstrates a locoregional control and overall survival (OS) benefit compared with radiation therapy alone in patients who have high-risk pathological risk factors, extracapsular extension (ECE) of a lymph node, or positive margins, based on a pooled analysis of the EORTC-22931 (NCT00002555) and RTOG-9501 (NCT00002670) studies.[7-10][Level of evidence A1]
For patients with intermediate pathological risk factors, the addition of cisplatin chemotherapy given concurrently with PORT is unclear. Intermediate pathological risk factors include:
- T3 and T4 disease (or stage III and stage IV disease).
- Perineural infiltration.
- Vascular embolisms.
- Clinically enlarged level IV–V lymph nodes secondary to tumors arising in the oral cavity or oropharynx.
- Two or more histopathologically involved lymph nodes without ECE.
- Close margins less than 5 mm.
The addition of cetuximab with radiation therapy in the postoperative setting for these intermediate pathological risk factors is being tested in a randomized trial (RTOG-0920 [NCT00956007]).
Radiation Therapy
A review of published clinical results of radiation therapy for head and neck cancer suggested a significant loss of local control when the administration of radiation therapy was prolonged. Therefore, extending standard treatment schedules is detrimental.[11,12]
Patients who are smokers appear to have lower response rates and shorter survival times than those who do not smoke while receiving radiation therapy.[13] Counseling patients to stop smoking before beginning radiation therapy may be beneficial.
Intensity-modulated radiation therapy (IMRT) has become a standard technique for head and neck radiation therapy. IMRT allows a dose-painting technique, also known as a simultaneous-integrated-boost (SIB) technique, with a dose per fraction slightly higher than 2 Gy, which allows slight shortening of overall treatment time and increases the biologically equivalent dose to the tumor.
Evidence (definitive radiation therapy):
- IMRT was studied in a phase II trial (RTOG-0022 [NCT00006360]) of 69 patients with stages T1 to T2, N0 to N1, M0 oropharyngeal carcinoma who were treated with primary radiation therapy without chemotherapy.[14] The median follow-up was 2.8 years. The prescribed planning target volume (PTV) dose to the primary tumor and involved nodes was 66 Gy at 2.2 Gy per fraction over 6 weeks. Subclinical PTVs received simultaneously 54 to 60 Gy at 1.8 to 2.0 Gy per fraction using an SIB technique. The following results were observed:
- The 2-year estimated locoregional failure rate was 9%. Two of four patients (50%), who had major underdose deviations, had locoregional failure compared with 3 of 49 patients (6%) without such deviations (P = .04).
- Maximal late toxicities with a grade of 2 or higher were skin (12%), mucosa (24%), salivary (67%), esophagus (19%), and osteoradionecrosis (6%).
- Longer follow-up revealed reduced late toxicity in all categories. Xerostomia grade 2 or higher was observed in 55% of patients at 6 months but was reduced to 25% of patients at 12 months and 16% of patients at 24 months.
The RTOG-0022 study showed high control rates and the feasibility of IMRT at a multi-institutional level; the study also showed high tumor control rates and reduced salivary toxicity compared with previous Radiation Therapy Oncology Group (RTOG) studies. However, major target underdose deviations were associated with a higher locoregional failure rate.
- Similar nonrandomized multicenter studies used fractionally escalated doses, ranging from 2.3 to 2.5 Gy with IMRT. These doses have been safe when given without concurrent chemotherapy for pharyngolaryngeal T2N0, T2N1, or laryngeal T3N0 SCC.[15-19]
- No toxicity difference was observed between the different dose-escalated groups.
- A randomized trial (PARSPORT [NCT00081029]) conducted in the United Kingdom compared conventional 3-dimensional conformal radiation therapy with IMRT. The following results were observed:[20][Level of evidence A1]
- The rate of xerostomia was significantly lower in the IMRT group than in the conventional group.
- Fatigue was more prevalent in the IMRT group.
- No significant differences were seen in nonxerostomia late toxicities, locoregional control, or OS at 24 months.
Altered fractionation versus standard fractionation radiation therapy
Radiation therapy alone with altered fractionation may be used for patients with locally advanced oropharyngeal cancer who are not candidates for chemotherapy. Altered fractionated radiation therapy yields a higher locoregional control rate than standard fractionated radiation therapy for patients with stage III or stage IV oropharyngeal cancer.
Evidence (altered fractionation vs. standard fractionation):
- The randomized RTOG-9003 trial (NCT00771641) included four radiation therapy treatment arms:[21,22][Level of evidence A1]
- Standard fractionation (SFX) to 70 Gy in 35 daily fractions for 7 weeks.
- Hyperfractionation (HFX) to 81.6 Gy in 68 twice-daily fractions for 7 weeks.
- Accelerated fractionation split course (AFX-S) to 67.2 Gy in 42 fractions for 6 weeks with a 2-week rest after 38.4 Gy.
- Accelerated concurrent boost fractionation (AFX-C) to 72 Gy in 42 fractions for 6 weeks.
In a long-term analysis, the three investigational arms were compared with SFX.
- Only the HFX arm showed superior locoregional control and survival at 5 years compared with the SFX arm (HR, 0.79; 95% CI, 0.62–1.00; P = .05).
- AFX-C was associated with increased late toxicity compared with SFX.
- The following results were shown in a meta-analysis of 15 randomized trials with a total of 6,515 patients and a median follow-up of 6 years involving the assessment of HFX or AFX-S for patients with stage III and stage IV oropharyngeal cancer:[23][Level of evidence A1]
- There was a significant survival benefit with altered-fractionated radiation therapy and a 3.4% absolute benefit at 5 years (HR, 0.92; 95% CI, 0.86–0.97; P = .003).
- Altered-fractionation radiation therapy improves locoregional control, with greater benefit shown in younger patients.
- HFX demonstrated a greater survival benefit (8% at 5 years) than did AFX-S (2% with accelerated fractionation without total dose-reduction and 1.7% with total dose-reduction at 5 years, P = .02).
An additional late effect from radiation therapy is hypothyroidism, which occurs in 30% to 40% of patients who have received external-beam radiation therapy to the entire thyroid gland. Thyroid function testing of patients is considered before therapy and as part of posttreatment follow-up.[24,25]
Prospective data of two randomized controlled trials reported the incidence of hypothyroidism.[26]
- At a median follow-up of 41 months, 55.1% of the patients developed hypothyroidism (39.3% subclinical, 15.7% biochemical).
- Patients who underwent IMRT had higher subclinical hypothyroidism (51.1% vs. 27.3%; P = .021), peaking around 1 year after radiation therapy.
- Younger age, hypopharynx/larynx primary, node positivity, higher dose/fraction (IMRT arm), and D100 were statistically significant factors for developing hypothyroidism.[26][Level of evidence A3]
For patients with well-lateralized oropharyngeal cancer, such as a T1 or T2 tonsil primary tumor with limited extension into the palate or tongue base, and limited ipsilateral lymph node involvement without extracapsular extension, elective treatment to the ipsilateral lymph nodes results in only minimal risk of spread to the contralateral neck.[27] For T3 and T4 tumors that are midline or approach the midline, bilateral nodal treatment is a consideration. In addition to the cervical lymph node chain, retropharyngeal lymph nodes can also be encompassed in the elective nodal treatment.
Concurrent Chemoradiation Therapy
Concurrent chemoradiation therapy is a standard treatment option for patients with locally advanced (stage III and stage IV) oropharyngeal carcinoma and is superior to radiation therapy alone.[28] This treatment approach emphasizes organ preservation and functionality.[29,30]
Evidence (concurrent chemoradiation therapy):
- A meta-analysis that originally included 93 randomized prospective head and neck cancer trials published between 1965 and
2000 was updated with the addition of 16 new trials (including 2,767 patients) and 11 updated trials. The results confirmed the benefit and superiority of the addition of concurrent chemotherapy for nonmetastatic head and neck cancer.[31,32][Level of evidence A2]
- The subset of patients who received chemotherapy and radiation therapy had a 6.5% absolute survival advantage, with OS increasing from 27.7% to 33.6% at 5 years and from 17.3% to 20.9% at 10 years.
- Patients who received concurrent chemotherapy had a greater survival benefit than did those who received neoadjuvant chemotherapy.
-
Postoperative chemoradiation therapy with cisplatin 100 mg/m2 given once every 3 weeks is standard treatment for patients with disease at high risk for recurrence, mainly those with extracapsular lymph node extension and positive surgical margins. However, this dosage has raised concerns about insufficient cisplatin delivery because of high-dose–related toxicity. Chemoradiation therapy with weekly cisplatin is widely used as an alternative with a better safety profile.
A multi-institutional, open-label, noninferiority, phase II/III trial compared different cisplatin schedules as part of postoperative treatment for patients with high-risk locally advanced SCC of the head and neck. Patients received either cisplatin (40 mg/m2) once weekly or standard-dose cisplatin (100 mg/m2) once every 3 weeks, both combined with radiation therapy. OS was the primary end point of the phase III portion of the study. An HR of 1.32 was set as the noninferiority margin. A total of 261 patients were enrolled (cisplatin every 3 weeks, 132 patients; cisplatin weekly, 129 patients).[33]
- At the planned third interim analysis in the phase III part of the trial, after a median follow-up of 2.2 years, chemoradiation therapy with weekly cisplatin was noninferior to cisplatin every 3 weeks in terms of OS (HR, 0.69; 99.1% CI, 0.374–1.273 [<1.32]; one-sided P for noninferiority = .0027).[33][Level of evidence A1]
- Grade 3 or 4 neutropenia and infection were less frequent in patients who received cisplatin weekly. Grade 3 or 4 neutropenia occurred in 49% of patients who received cisplatin every 3 weeks and 35% of patients who received cisplatin weekly. Grade 3 or 4 infection occurred in 12% of patients who received cisplatin every 3 weeks and 7% of patients who received cisplatin weekly. Grade 3 or 4 renal impairment and hearing impairment were also less frequent in patients who received cisplatin weekly. No treatment-related deaths were reported among patients who received cisplatin every 3 weeks, and two deaths were reported among patients who received weekly cisplatin (1.6%).
Regimens with cisplatin given weekly or every 3 weeks are both considered standard care. A large, randomized, prospective trial is evaluating the equivalent efficacy of these regimens.
- A phase III randomized trial in India included patients with locally advanced SCC of the head and neck unsuitable for cisplatin-based chemoradiation. This study evaluated using docetaxel as a radiosensitizer. Patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2 were randomly assigned (1:1) to receive either radiation therapy alone or with concurrent docetaxel (15 mg/m2) once weekly for a maximum of seven cycles. The primary end point was 2-year disease-free survival (DFS). Most patients (about 60%) received the treatment as definitive. Cisplatin ineligibility was defined by multiple parameters, including an ECOG PS of 2, calculated creatinine clearance <50 mL/min, and organ dysfunction of grade 2 or higher. A total of 356 patients were enrolled (176 in the radiation therapy arm and 180 in the radiation-and-docetaxel arm).[34]
- The 2-year DFS rate was 30.3% in the radiation-alone arm (95% CI, 23.6%–37.4%) and 42% in the radiation-and-docetaxel arm (95% CI, 34.6%–49.2%) (HR, 0.673; 95% CI, 0.521–0.868; P = .002).
- The corresponding median OS was 15.3 months in the radiation-alone arm (95% CI, 13.1–22.0) and 25.5 months in the radiation-and-docetaxel arm (95% CI, 17.6–32.5) (log-rank P = .035). The 2-year OS rate was 41.7% in the radiation-alone arm (95% CI, 34.1%–49.1%) and 50.8% in the radiation-and-docetaxel arm (95% CI, 43.1%–58.1%) (HR, 0.747; 95% CI, 0.569–0.980; P = .035).[34][Level of evidence A1]
- There was a higher incidence of grade 3 or higher mucositis (22.2% vs. 49.7%; P < .001), odynophagia (33.5% vs. 52.5%; P < .001), and dysphagia (33% vs. 49.7%; P = .002) for patients who received radiation and docetaxel versus radiation alone.
- The increase of toxicity observed in the radiation-and-docetaxel arm did not significantly impair the total radiation dose the patients received. A total of 88.9% of patients in the radiation-and-docetaxel arm and 93.8% of patients in the radiation-alone arm received the total radiation dose. In the radiation-and-docetaxel arm, 85.6% of patients received five or more cycles of chemotherapy.
- In a randomized trial of patients with locally advanced head and neck cancer, curative-intent radiation therapy alone (213 patients) was compared with radiation therapy plus weekly cetuximab (211 patients).[35] The initial dose of cetuximab was 400 mg/m2 of body-surface area 1 week before radiation therapy was started, followed by a weekly dose of 250 mg/m2 of body-surface area for the duration of the radiation therapy. This study allowed altered-fractionation regimens to be used in both arms.[35,36][Level of evidence A1]
- At a median follow-up of 54 months, patients treated with cetuximab and radiation therapy demonstrated significantly higher progression-free survival (PFS) (HRdeath or for disease progression, 0.70; P = .006).
- Patients in the cetuximab arm experienced higher rates of acneiform rash and infusion reactions, although the incidence of other grade 3 or higher toxicities, including mucositis, did not differ significantly between the two groups.
Cetuximab versus cisplatin in patients with human papillomavirus (HPV)-positive oropharyngeal cancer
Studies evaluating de-intensification using reduced-dose radiation therapy (NRG-HN002 [NCT02254278] and ECOG-3311 [NCT01898494]) are ongoing in patients with low-risk HPV-positive oropharyngeal cancer. Cetuximab, an epidermal growth factor receptor inhibitor, has been evaluated in two randomized trials as a proposed de-intensification strategy to reduce the toxicity of cisplatin-based treatment.
Evidence (cetuximab versus cisplatin in patients with HPV-positive oropharyngeal cancer):
- In the randomized RTOG-1016 trial (NCT01302834), patients with HPV-positive (determined by central confirmation of p16 immunohistochemistry) oropharyngeal cancer were randomly assigned (1:1) to receive either radiation therapy with cetuximab or radiation therapy with cisplatin. This trial aimed to determine whether treatment with radiation therapy and cetuximab produced noninferior survival compared with treatment using radiation therapy and cisplatin. Of the 987 patients enrolled, 849 were randomly assigned to receive radiation therapy plus cetuximab (n = 425) or radiation therapy plus cisplatin (n = 424). Subsequently, 399 patients assigned to receive cetuximab and 406 patients assigned to receive cisplatin were determined to be eligible. Patients received 70 Gy of radiation therapy in 6 weeks accelerated (six fractions/week) with either two cycles of cisplatin (100 mg/m2) every 3 weeks or weekly cetuximab.[37][Level of evidence A1]
- After a median follow-up duration of 4.5 years, radiation therapy plus cetuximab did not meet the noninferiority criteria for OS (HR, 1.45; one-sided 95% upper CI, 1.94; P = .5056 for noninferiority; one-sided log-rank P = .0163).[38][Level of evidence A1]
- The estimated 5-year OS rate was 77.9% (95% CI, 73.4%–82.5%) in the cetuximab group versus 84.6% (80.6%–88.6%) in the cisplatin group.
- PFS was significantly lower in the cetuximab group than in the cisplatin group (HR, 1.72; 95% CI, 1.29–2.29; P = .0002; 5-year PFS rate 67.3%; 95% CI, 62.4%–72.2% vs. 78.4%, 73.8%–83.0%), and locoregional failure was significantly higher in the cetuximab group than in the cisplatin group.
- Acute moderate to severe toxicity (77.4%, 95% CI, 73.0%–81.5% vs. 81.7%, 77.5%–85.3%; P = .1586) and late moderate to severe toxicity (16.5%, 95% CI, 12.9%–20.7% vs. 20.4%, 16.4%–24.8%; P = .1904) were similar between the cetuximab and cisplatin groups.
- De-ESCALaTE HPV [NCT01874171] was an open-label, randomized, controlled, phase III trial at 32 head and neck treatment centers in Ireland, the Netherlands, and the United Kingdom. It included patients aged 18 years or older with HPV-positive, low-risk oropharyngeal cancer (nonsmokers or lifetime smokers with a smoking history of <10 pack-years).[39] Patients were randomly assigned (1:1) to receive, in addition to radiation therapy (70 Gy in 35 fractions), either intravenous cisplatin (100 mg/m2 on days 1, 22, and 43 of radiation therapy) or intravenous cetuximab (400 mg/m2 loading dose followed by seven weekly infusions of 250 mg/m2).
The primary outcome was overall severe (grades 3–5) toxicity events at 24 months from the end of treatment. The primary outcome was assessed by intention-to-treat and per-protocol analyses. Between Nov 12, 2012, and Oct 1, 2016, 334 patients were recruited (166 in the cisplatin group and 168 in the cetuximab group).
- Overall (acute and late) severe (grades 3–5) toxicity did not differ significantly between treatment groups at 24 months (mean number of events per patient, 4.8 [95% CI, 4.2–5.4] with cisplatin vs. 4.8 [4.2–5.4] with cetuximab; P = .98).[39][Level of evidence A1]
- At 24 months, overall all-grade toxicity did not differ significantly (mean number of events per patient, 29.2 [95% CI, 27.3–31.0] with cisplatin vs. 30.1 [28.3–31.9] with cetuximab; P = .49).
- OS was inferior and local recurrence rate was higher in the cetuximab arm. The 2-year OS rate was 97.5% for the cisplatin group versus 89.4% for the cetuximab group (HR, 5.0; 95% CI, 1.7–14.7; P = .001), and the 2-year recurrence rate was 6.0% for the cisplatin group versus 16.1% for the cetuximab group (HR, 3.4; 1.6–7.2; P = .0007).
These findings showed the inferiority of cetuximab compared with cisplatin for OS and local recurrence rates for patients with locoregionally advanced HPV-related oropharyngeal cancer and also did not demonstrate reduced toxicity with cetuximab and radiation therapy compared with cisplatin. Treatment with the combination of radiation therapy and cetuximab resulted in inferior OS and PFS compared with treatment using radiation therapy and cisplatin; therefore, treatment with radiation therapy and cisplatin remains the standard of care.
For more information about oral toxicities, see Oral Complications of Cancer Therapies.
Neoadjuvant Chemotherapy Followed by Concurrent Chemoradiation Therapy
In a meta-analysis of five randomized trials, a total of 1,022 patients with locally advanced head and neck SCC were assigned to receive either neoadjuvant chemotherapy with TPF (docetaxel, cisplatin, and fluorouracil) followed by concurrent chemoradiation therapy or concurrent chemoradiation therapy alone. The analysis failed to show an OS (HR, 1.01; 95% confidence limits [CLs], 0.84, 1.21; P = .92) or PFS (HR, 0.91; 95% CLs, 0.75, 1.1; P = .32) advantage for neoadjuvant chemotherapy using the TPF regimen over concurrent chemoradiation therapy alone.[40][Level of evidence A1]
Evidence (neoadjuvant chemotherapy followed by concurrent chemoradiation therapy):
- In a phase II study of neoadjuvant chemotherapy using cisplatin, paclitaxel, and cetuximab in patients with HPV-associated oropharyngeal SCC (ECOG 1308 [NCT01084083]), patients who achieved a complete clinical response to three cycles of neoadjuvant chemotherapy received reduced-dose IMRT of 54 Gy with weekly cetuximab. Patients with less than a clinical complete response received 69.3 Gy of radiation to the primary site or nodes and cetuximab.[41]
- With a median follow-up of 35.4 months, the 2-year PFS rate was 80% and the OS rate was 94% for patients who achieved a complete clinical response and were treated with 54 Gy of radiation.
- For patients whose primary tumor (T) was less than T4 and regional lymph nodes (N) were less than N2c and had a smoking history of less than 10 pack-years, the 2-year PFS rate was 96% and the OS rate was 96%.
- Lower-dose radiation using 54 Gy was associated with lower rates of dysphagia with solid foods and less-impaired nutrition.
- There does not appear to be a benefit from treatment with neoadjuvant chemotherapy followed by full-dose (≥70 Gy) concurrent chemoradiation therapy. There may be a role for radiation dose de-escalation in HPV-positive patients with low-risk disease who achieve a complete clinical response after neoadjuvant chemotherapy.
Overall, the role of neoadjuvant chemotherapy for patients with oropharyngeal cancer remains unclear. However, in HPV-defined subsets, more information is needed because, as this phase II study suggests, in that setting, neoadjuvant chemotherapy may be used with less chemoradiation.[40,42-45][Level of evidence A1]
Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[46,47] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[46-48] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[49-51] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[52] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[53]
References
- Iyer NG, Tan DS, Tan VK, et al.: Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10-year update and subset analysis. Cancer 121 (10): 1599-607, 2015. [PUBMED Abstract]
- Mehanna H, Wong WL, McConkey CC, et al.: PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N Engl J Med 374 (15): 1444-54, 2016. [PUBMED Abstract]
- Parsons JT, Mendenhall WM, Stringer SP, et al.: Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer 94 (11): 2967-80, 2002. [PUBMED Abstract]
- Harrison LB, Zelefsky MJ, Armstrong JG, et al.: Performance status after treatment for squamous cell cancer of the base of tongue--a comparison of primary radiation therapy versus primary surgery. Int J Radiat Oncol Biol Phys 30 (4): 953-7, 1994. [PUBMED Abstract]
- Mendenhall WM, Morris CG, Amdur RJ, et al.: Definitive radiotherapy for squamous cell carcinoma of the base of tongue. Am J Clin Oncol 29 (1): 32-9, 2006. [PUBMED Abstract]
- Chen AM, Daly ME, Luu Q, et al.: Comparison of functional outcomes and quality of life between transoral surgery and definitive chemoradiotherapy for oropharyngeal cancer. Head Neck 37 (3): 381-5, 2015. [PUBMED Abstract]
- Cooper JS, Pajak TF, Forastiere AA, et al.: Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350 (19): 1937-44, 2004. [PUBMED Abstract]
- Bernier J, Domenge C, Ozsahin M, et al.: Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350 (19): 1945-52, 2004. [PUBMED Abstract]
- Bernier J, Cooper JS, Pajak TF, et al.: Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 27 (10): 843-50, 2005. [PUBMED Abstract]
- Cooper JS, Zhang Q, Pajak TF, et al.: Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 84 (5): 1198-205, 2012. [PUBMED Abstract]
- Fowler JF, Lindstrom MJ: Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phys 23 (2): 457-67, 1992. [PUBMED Abstract]
- Allal AS, de Pree C, Dulguerov P, et al.: Avoidance of treatment interruption: an unrecognized benefit of accelerated radiotherapy in oropharyngeal carcinomas? Int J Radiat Oncol Biol Phys 45 (1): 41-5, 1999. [PUBMED Abstract]
- Browman GP, Wong G, Hodson I, et al.: Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 328 (3): 159-63, 1993. [PUBMED Abstract]
- Eisbruch A, Harris J, Garden AS, et al.: Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys 76 (5): 1333-8, 2010. [PUBMED Abstract]
- Leclerc M, Maingon P, Hamoir M, et al.: A dose escalation study with intensity modulated radiation therapy (IMRT) in T2N0, T2N1, T3N0 squamous cell carcinomas (SCC) of the oropharynx, larynx and hypopharynx using a simultaneous integrated boost (SIB) approach. Radiother Oncol 106 (3): 333-40, 2013. [PUBMED Abstract]
- Buettner F, Miah AB, Gulliford SL, et al.: Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol 103 (1): 82-7, 2012. [PUBMED Abstract]
- Gulliford SL, Miah AB, Brennan S, et al.: Dosimetric explanations of fatigue in head and neck radiotherapy: an analysis from the PARSPORT Phase III trial. Radiother Oncol 104 (2): 205-12, 2012. [PUBMED Abstract]
- Kohler RE, Sheets NC, Wheeler SB, et al.: Two-year and lifetime cost-effectiveness of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 87 (4): 683-9, 2013. [PUBMED Abstract]
- Gupta T, Agarwal J, Jain S, et al.: Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol 104 (3): 343-8, 2012. [PUBMED Abstract]
- Nutting CM, Morden JP, Harrington KJ, et al.: Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 12 (2): 127-36, 2011. [PUBMED Abstract]
- Fu KK, Pajak TF, Trotti A, et al.: A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 48 (1): 7-16, 2000. [PUBMED Abstract]
- Beitler JJ, Zhang Q, Fu KK, et al.: Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 89 (1): 13-20, 2014. [PUBMED Abstract]
- Baujat B, Bourhis J, Blanchard P, et al.: Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev (12): CD002026, 2010. [PUBMED Abstract]
- Turner SL, Tiver KW, Boyages SC: Thyroid dysfunction following radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 31 (2): 279-83, 1995. [PUBMED Abstract]
- Constine LS: What else don't we know about the late effects of radiation in patients treated for head and neck cancer? Int J Radiat Oncol Biol Phys 31 (2): 427-9, 1995. [PUBMED Abstract]
- Murthy V, Narang K, Ghosh-Laskar S, et al.: Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck 36 (11): 1573-80, 2014. [PUBMED Abstract]
- O'Sullivan B, Warde P, Grice B, et al.: The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys 51 (2): 332-43, 2001. [PUBMED Abstract]
- Denis F, Garaud P, Bardet E, et al.: Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22 (1): 69-76, 2004. [PUBMED Abstract]
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Adelstein DJ: Oropharyngeal cancer: the role of chemotherapy. Curr Treat Options Oncol 4 (1): 3-13, 2003. [PUBMED Abstract]
- Pignon JP, le Maître A, Maillard E, et al.: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92 (1): 4-14, 2009. [PUBMED Abstract]
- Lacas B, Carmel A, Landais C, et al.: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol 156: 281-293, 2021. [PUBMED Abstract]
- Kiyota N, Tahara M, Mizusawa J, et al.: Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J Clin Oncol 40 (18): 1980-1990, 2022. [PUBMED Abstract]
- Patil VM, Noronha V, Menon N, et al.: Results of Phase III Randomized Trial for Use of Docetaxel as a Radiosensitizer in Patients With Head and Neck Cancer, Unsuitable for Cisplatin-Based Chemoradiation. J Clin Oncol 41 (13): 2350-2361, 2023. [PUBMED Abstract]
- Bonner JA, Harari PM, Giralt J, et al.: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354 (6): 567-78, 2006. [PUBMED Abstract]
- Curran D, Giralt J, Harari PM, et al.: Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 25 (16): 2191-7, 2007. [PUBMED Abstract]
- Trotti A, Harris J, Gillison M, et al.: NRG-RTOG 1016: phase III trial comparing radiation/cetuximab to radiation/cisplatin in HPV-related cancer of the oropharynx. [Abstract] Int J Radiat Oncol Biol Phys 102 (5): A-LBA4, 1604-5, 2018. Also available online. Last accessed November 18, 2024.
- Gillison ML, Trotti AM, Harris J, et al.: Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393 (10166): 40-50, 2019. [PUBMED Abstract]
- Mehanna H, Robinson M, Hartley A, et al.: Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393 (10166): 51-60, 2019. [PUBMED Abstract]
- Budach W, Bölke E, Kammers K, et al.: Induction chemotherapy followed by concurrent radio-chemotherapy versus concurrent radio-chemotherapy alone as treatment of locally advanced squamous cell carcinoma of the head and neck (HNSCC): A meta-analysis of randomized trials. Radiother Oncol 118 (2): 238-43, 2016. [PUBMED Abstract]
- Marur S, Li S, Cmelak AJ, et al.: E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol 35 (5): 490-497, 2017. [PUBMED Abstract]
- Haddad R, O'Neill A, Rabinowits G, et al.: Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 14 (3): 257-64, 2013. [PUBMED Abstract]
- Cohen EE, Karrison TG, Kocherginsky M, et al.: Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 32 (25): 2735-43, 2014. [PUBMED Abstract]
- Hitt R, Grau JJ, López-Pousa A, et al.: A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25 (1): 216-25, 2014. [PUBMED Abstract]
- Driessen CM, de Boer JP, Gelderblom H, et al.: Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil followed by randomization to two cisplatin-based concomitant chemoradiotherapy schedules in patients with locally advanced head and neck cancer (CONDOR study) (Dutch Head and Neck Society 08-01): A randomized phase II study. Eur J Cancer 52: 77-84, 2016. [PUBMED Abstract]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
Treatment of Stage I and Stage II Oropharyngeal Cancer
Treatment Options for Stage I and Stage II Oropharyngeal Cancer
The management of stage I and stage II carcinomas of the oropharynx requires multidisciplinary input to establish the optimal treatment. Radiation therapy or surgery is equally successful in controlling stage I and stage II oropharyngeal cancer. For more information, see the Treatment Option Overview for Oropharyngeal Cancer section.
The choice of treatment is dictated by the anticipated functional speech and swallowing and cosmetic outcome of the treatment options and by the available expertise of the surgeon or radiation oncologist. Treatment is individualized for each patient.
Treatment options for stage I and stage II oropharyngeal cancer include:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of Stage III and Stage IV Oropharyngeal Cancer
The management of stage III and stage IV carcinomas of the oropharynx is complex and requires multidisciplinary input to establish the optimal treatment. For more information, see the Treatment Option Overview for Oropharyngeal Cancer section.
Treatment Options for Stage III and Stage IV Oropharyngeal Cancer
Treatment options for stage III and stage IV oropharyngeal cancer include:
- Surgery and postoperative radiation therapy (PORT) with or without chemotherapy for patients with advanced disease.[1-4][Level of evidence A1]
- Radiation therapy using altered fractionation.[5-9][Level of evidence A1]
- Concurrent chemoradiation therapy.[10-15][Level of evidence A1]
- Neoadjuvant chemotherapy followed by concurrent chemoradiation therapy.
- Chemoradiation therapy with immunotherapy (under clinical evaluation). RTOG 3504 (NCT02764593) evaluated concurrent chemoradiation therapy with nivolumab in patients with intermediate- to high-risk head and neck cancer.
- Treatment de-intensification using radiation dose de-escalation is being studied in NRG-HN002 (NCT02254278).
- Treatment de-intensification using transoral surgery followed by radiation dose de-escalation is being studied in ECOG-3311 (NCT01898494).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bernier J, Cooper JS, Pajak TF, et al.: Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 27 (10): 843-50, 2005. [PUBMED Abstract]
- Cooper JS, Zhang Q, Pajak TF, et al.: Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 84 (5): 1198-205, 2012. [PUBMED Abstract]
- Cooper JS, Pajak TF, Forastiere AA, et al.: Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350 (19): 1937-44, 2004. [PUBMED Abstract]
- Bernier J, Domenge C, Ozsahin M, et al.: Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350 (19): 1945-52, 2004. [PUBMED Abstract]
- Horiot JC, Le Fur R, N'Guyen T, et al.: Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol 25 (4): 231-41, 1992. [PUBMED Abstract]
- Bourhis J, Lapeyre M, Tortochaux J, et al.: Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. J Clin Oncol 24 (18): 2873-8, 2006. [PUBMED Abstract]
- Overgaard J, Hansen HS, Specht L, et al.: Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 362 (9388): 933-40, 2003. [PUBMED Abstract]
- Overgaard J, Mohanti BK, Begum N, et al.: Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial. Lancet Oncol 11 (6): 553-60, 2010. [PUBMED Abstract]
- Fu KK, Pajak TF, Trotti A, et al.: A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 48 (1): 7-16, 2000. [PUBMED Abstract]
- Bonner JA, Harari PM, Giralt J, et al.: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354 (6): 567-78, 2006. [PUBMED Abstract]
- Curran D, Giralt J, Harari PM, et al.: Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 25 (16): 2191-7, 2007. [PUBMED Abstract]
- Denis F, Garaud P, Bardet E, et al.: Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22 (1): 69-76, 2004. [PUBMED Abstract]
- Olmi P, Crispino S, Fallai C, et al.: Locoregionally advanced carcinoma of the oropharynx: conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy--a multicenter randomized trial. Int J Radiat Oncol Biol Phys 55 (1): 78-92, 2003. [PUBMED Abstract]
- Semrau R, Mueller RP, Stuetzer H, et al.: Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 64 (5): 1308-16, 2006. [PUBMED Abstract]
- Pignon JP, le Maître A, Maillard E, et al.: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92 (1): 4-14, 2009. [PUBMED Abstract]
Treatment of Metastatic and Recurrent Oropharyngeal Cancer
Treatment Options for Metastatic and Recurrent Oropharyngeal Cancer
The management of metastatic and recurrent carcinomas of the oropharynx is complex and requires multidisciplinary input to establish the optimal treatment. For more information, see the Treatment Option Overview for Oropharyngeal Cancer section.
Treatment options for metastatic and recurrent oropharyngeal cancer include:
- Surgical resection, if technically feasible and the tumor does not respond to radiation therapy.[1]
- Radiation therapy, if the tumor is not completely removed by surgery and curative doses of radiation have not been given previously.[2]
- A second surgery, if the tumor was not completely removed initially and if technically feasible.[1]
- Chemotherapy, for unresectable locoregionally recurrent disease.
- Additional radiation therapy using conventionally fractionated radiation therapy, or hyperfractionated radiation therapy (HFX) with concurrent chemotherapy.[3]
- Stereotactic body radiation therapy with concurrent cetuximab.[4]
- Immunotherapy (inhibitor of the programmed death-ligand 1 [PD-L1] pathway) can be used after platinum-based chemotherapy failure [5,6] or up front in patients with metastatic or locally recurrent disease.[7,8]
- Clinical trials evaluating additional radiation therapy using HFX with concurrent chemotherapy, targeted therapy, stereotactic body radiation therapy, or immunotherapy.[9]
Chemotherapy
Platinum-based chemotherapy is often used as first-line treatment for patients with metastatic or recurrent squamous cell carcinoma (SCC) of the head and neck.
Evidence (chemotherapy):
- In a phase III randomized trial of 442 patients with untreated metastatic or recurrent SCC of the head and neck, adding cetuximab to platinum plus fluorouracil (5-FU) improved overall survival (OS), compared with platinum plus 5-FU alone. The median survival was 10.1 months versus 7.4 months (hazard ratio [HR]death, 0.80; 95% confidence interval [CI], 0.64–0.99; P = .04).[10]
- Quality of life (QOL) was not adversely affected by adding cetuximab to this platinum-based regimen.[11]
Tumor EGFR gene copy number was not a predictive biomarker for the efficacy of cetuximab plus platinum and 5-FU as first-line therapy for patients with metastatic or recurrent SCC of the head and neck.[12][Level of evidence A1]
- An open-label, phase III, randomized trial demonstrated improved progression-free survival (PFS) for patients who received afatinib compared with patients who received methotrexate.[13]
- After a median follow-up of 6.7 months, the median PFS was 2.6 months (95% CI, 2.0–2.7) for the afatinib group and 1.7 months (95% CI, 1.5–2.4) for the methotrexate group (HR, 0.80; 95% CI, 0.65–0.98; P = .030).
- The most frequent grade 3 or grade 4 drug-related adverse events for patients treated with afatinib or methotrexate included:
- Rash or acne (10% for afatinib vs. 0% for methotrexate).
- Diarrhea (9% for afatinib vs. 2% for methotrexate).
- Stomatitis (6% for afatinib vs. 8% for methotrexate).
- Fatigue (6% for afatinib vs. 3% for methotrexate).
- Neutropenia (<1% for afatinib vs. 7% for methotrexate).
- Overall, serious adverse events occurred in 14% of patients treated with afatinib and 11% of patients treated with methotrexate.
Immunotherapy
Pembrolizumab
Pembrolizumab is a monoclonal antibody and an inhibitor of the programmed death-1 (PD-1) pathway. Studies have evaluated pembrolizumab in patients with incurable metastatic or recurrent head and neck squamous cell carcinoma (SCC).
Evidence (pembrolizumab as first-line therapy):
- KEYNOTE-048 (NCT02358031) was a nonblinded, randomized, phase III study of participants with untreated locally incurable metastatic or recurrent head and neck SCC that was performed at 200 sites in 37 countries.[7] A total of 882 patients were randomly assigned in a 1:1:1 ratio to receive pembrolizumab alone (n = 301), pembrolizumab plus a platinum and fluorouracil (5-FU) (pembrolizumab with chemotherapy) (n = 281), or cetuximab plus a platinum and 5-FU (cetuximab with chemotherapy) (n = 300). Investigators, patients, and representatives of the sponsor were masked to the programmed death-ligand 1 (PD-L1) combined positive score (CPS) results; PD-L1 positivity was not required for study entry. A total of 754 patients (85%) had a CPS of 1 or higher and 381 patients (43%) had a CPS of 20 or higher.
The primary end points were overall survival (OS) and progression-free survival (PFS). Progression was defined as radiographically confirmed disease progression or death from any cause, whichever came first, in the intention-to-treat population.
- At the second interim analysis, pembrolizumab alone showed improved or noninferior OS compared with cetuximab with chemotherapy. The median OS results were reported as follows:[7][Level of evidence A1]
- Among the population with a CPS of 20 or higher, the median OS was 14.9 months in patients who received pembrolizumab alone and 10.7 months in patients who received cetuximab with chemotherapy (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.45–0.83; P = .0007).
- Among the population with a CPS of 1 or higher, the median OS was 12.3 months in patients who received pembrolizumab alone and 10.3 months in patients who received cetuximab with chemotherapy (HR, 0.78; 95% CI, 0.64–0.96; P = .0086).
- Among the total population, patients who received pembrolizumab alone had noninferior OS (11.6 months) compared with patients who received cetuximab with chemotherapy (10.7 months) (HR, 0.85; 95% CI, 0.71–1.03; P = .0456).
- Pembrolizumab with chemotherapy showed improved OS versus cetuximab with chemotherapy. The OS results were reported as follows:
- At the second interim analysis, among the total population, the median OS was 13.0 months in patients who received pembrolizumab with chemotherapy and 10.7 months in patients who received cetuximab with chemotherapy (HR, 0.77; 95% CI, 0.63–0.93; P = .0034).
- At the final analysis, among the population with a CPS of 20 or higher, the median OS was 14.7 months in patients who received pembrolizumab with chemotherapy and 11.0 months in patients who received cetuximab with chemotherapy (HR, 0.60; 95% CI, 0.45–0.82; P = .0004).
- At the final analysis, among the population with a CPS of 1 or higher, the median OS was 13.6 months in patients who received pembrolizumab with chemotherapy and 10.4 months in patients who received cetuximab with chemotherapy (HR, 0.65; 95% CI, 0.53–0.80; P < .0001).
- At the second interim analysis, neither pembrolizumab alone nor pembrolizumab with chemotherapy improved PFS.
- At the final analysis, grade 3 or higher all-cause adverse events occurred in 164 of 300 patients (55%) in the pembrolizumab-alone group, 235 of 276 patients (85%) who received pembrolizumab with chemotherapy, and 239 of 287 patients (83%) who received cetuximab with chemotherapy.
- Adverse events led to death in 25 patients (8%) in the pembrolizumab-alone group, 32 patients (12%) who received pembrolizumab with chemotherapy, and 28 patients (10%) who received cetuximab with chemotherapy.
- At the second interim analysis, pembrolizumab alone showed improved or noninferior OS compared with cetuximab with chemotherapy. The median OS results were reported as follows:[7][Level of evidence A1]
Pembrolizumab plus a platinum and 5-FU is an appropriate first-line treatment for patients with metastatic or recurrent head and neck SCC. Pembrolizumab monotherapy is an appropriate first-line treatment for patients with PD-L1–positive metastatic or recurrent head and neck SCC. These results were confirmed at a longer median follow-up of 45 months (interquartile range, 41.0–49.2).[8]
Evidence (pembrolizumab after progression on platinum-based treatment):
- The phase III KEYNOTE-040 (NCT02252042) trial included patients with incurable metastatic or recurrent head and neck SCC who had received platinum-based treatment within 3 to 6 months.[5] Patients were randomly assigned to the pembrolizumab arm (200 mg every 3 weeks [247 patients]) or to the standard therapy arm of the investigator’s choice (methotrexate, docetaxel, or cetuximab [248 patients]). Patients received treatment until progression or toxicity. The maximum duration of pembrolizumab was 24 months. The primary end point was OS in the intention-to-treat population.
- The median OS was 8.4 months in the pembrolizumab arm and 6.9 months in the standard therapy arm (HR, 0.80; 95% CI, 0.65–0.98; nominal P = .0161).[5][Level of evidence A1]
- Pembrolizumab was associated with fewer grade 3 or higher adverse events (pembrolizumab, 13% vs. standard therapy, 36%). The most common treatment-related adverse events were hypothyroidism (13%) in the pembrolizumab arm and fatigue (18%) in the standard therapy arm.
- In patients who received pembrolizumab, there were four treatment-related deaths resulting from large intestinal perforation, Stevens-Johnson syndrome, and unspecified malignant progression. Two treatment-related deaths in the standard therapy arm resulted from malignant progression and pneumonia.
- The PD-L1 CPS was 1 or higher in 79% of the patients in the pembrolizumab arm and 77% of the patients in the standard therapy arm.
- Compared with patients treated with standard therapy, a reduced HRdeath was noted for patients who received pembrolizumab and had PD-1 expression on their tumors or in the tumor microenvironment as noted by a PD-L1 CPS of 1 or higher (HR, 0.74; 95% CI, 0.58–0.93; nominal P = .0049) or a PD-L1 tumor proportion score of 50% or higher (HR, 0.53; 95% CI, 0.35–0.81; nominal P = .0014).
Nivolumab
Nivolumab is a fully human immunoglobulin G4 anti–PD-1 monoclonal antibody.
Evidence (nivolumab combined with ipilimumab in patients who have not previously received systemic therapy):
- The CheckMate 651 trial (NCT02741570) evaluated first-line nivolumab plus ipilimumab versus EXTREME (cetuximab, cisplatin/carboplatin, and 5-FU for up to six cycles followed by cetuximab maintenance) in patients with recurrent or metastatic head and neck SCC.[14] The primary end points were OS in all randomly assigned patients and patients with a PD-L1 CPS of 20 or higher. Secondary end points included OS in patients with a PD-L1 CPS of 1 or higher and PFS, objective response rate, and duration of response in all randomly assigned patients and patients with a PD-L1 CPS of 20 or higher.
- Among all randomly assigned patients, there was no statistically significant difference in OS with nivolumab plus ipilimumab versus EXTREME (median OS, 13.9 vs. 13.5 months; HR, 0.95; 97.9% CI, 0.80–1.13; P = .4951). Among patients with a PD-L1 CPS of 20 or higher, there was also no statistically significant OS difference between the two treatments (median OS, 17.6 vs. 14.6 months; HR, 0.78; 97.51% CI, 0.59–1.03; P = .0469).[14][Level of evidence A1]
- In patients with a CPS of 1 or higher, the median OS was 15.7 months for patients who received nivolumab plus ipilimumab versus 13.2 months for patients who received EXTREME (HR, 0.82; 95% CI, 0.69–0.97).
- Among patients with a CPS of 20 or higher, the median PFS was 5.4 months for patients who received nivolumab plus ipilimumab and 7.0 months for patients who received EXTREME. The objective response rate was 34.1% for patients who received nivolumab plus ipilimumab and 36.0% for patients who received EXTREME.
- Grade 3 or 4 treatment-related adverse events occurred in 28.2% of patients who received nivolumab plus ipilimumab and 70.7% of patients who received EXTREME.
- CheckMate 651 did not meet its primary end points of OS in the randomly assigned or CPS of 20 or higher populations.
The absence of a survival benefit for immune checkpoint inhibitors in this trial was an unexpected outcome, given the similarity of nivolumab to pembrolizumab in the studies of patients with cisplatin-refractory disease.[5,6] An editorial accompanying the CheckMate 651 trial analyzed some of the factors that may have contributed to a different result. The editorial suggested that survival in the control group, which was longer than that reported in prior studies, may have been impacted by the greater availability of second-line immunotherapy in the control group (46% in CheckMate 651 compared with 25% in the KEYNOTE-048 trial). The authors also suggested that the coadministration of ipilimumab detracted from the activity of nivolumab, as shown in the CheckMate 714 trial.[15]
- CheckMate 714 (NCT02823574), a double-blind phase II trial, evaluated the clinical benefit of first-line nivolumab plus ipilimumab versus nivolumab alone in 425 patients with recurrent or metastatic head and neck SCC.[16] Patients were randomly assigned in a 2:1 ratio to receive either nivolumab (3 mg/kg intravenously [IV] every 2 weeks) plus ipilimumab (1 mg/kg IV every 6 weeks) or nivolumab (3 mg/kg IV every 2 weeks) plus placebo. Treatment continued for up to 2 years or until disease progression, unacceptable toxic effects, or consent withdrawal. The primary end points were objective response rate and duration of response between treatment arms by blinded independent central review in the population with platinum-refractory recurrent or metastatic disease. These were patients who had recurrent disease less than 6 months after completion of platinum-based chemotherapy (adjuvant or neoadjuvant, or as part of multimodal treatment [chemotherapy, surgery, and/or radiation therapy]). Among the 241 patients (56.7%) with platinum-refractory disease, 159 were assigned to receive nivolumab plus ipilimumab and 82 were assigned to receive nivolumab alone. Among the 184 patients (43.3%) with platinum-eligible disease, 123 were assigned to receive nivolumab plus ipilimumab and 61 were assigned to receive nivolumab alone.[16][Level of evidence B3]
- At primary database lock, the objective response rate in the population with platinum-refractory disease was 13.2% (95% CI, 8.4%–19.5%) with nivolumab plus ipilimumab and 18.3% (95% CI, 10.6%–28.4%) with nivolumab alone (odds ratio, 0.68; 95.5% CI, 0.33–1.43; P = .29).
- The median duration of response was not reached (NR) in the nivolumab-plus-ipilimumab group (95% CI, 11.0 months–NR) and was 11.1 months (95% CI, 4.1–NR) in the nivolumab-alone group. In the population with platinum-eligible disease, the objective response rate was 20.3% (95% CI, 13.6%–28.5%) with nivolumab plus ipilimumab and 29.5% (95% CI, 18.5%–42.6%) with nivolumab alone.
- Among the population with platinum-refractory disease, grade 3 or 4 treatment-related adverse events occurred in 25 of 158 patients (15.8%) who received nivolumab plus ipilimumab and in 12 of 82 patients (14.6%) who received nivolumab alone. Among the population with platinum-eligible disease, grade 3 or 4 treatment-related adverse events occurred in 30 of 122 patients (24.6%) who received nivolumab plus ipilimumab and in 8 of 61 patients (13.1%) who received nivolumab alone.
- This trial did not meet its primary end point of objective response rate benefit with first-line nivolumab plus ipilimumab versus nivolumab alone in patients with platinum-refractory recurrent or metastatic head and neck SCC.
Evidence (nivolumab after progression on platinum-based treatment):
- A phase III open-label trial included 361 patients with recurrent SCC of the head and neck and disease progression within 6 months after platinum-based chemotherapy. Patients were randomly assigned in a 2:1 ratio to receive either nivolumab (at a dose of 3 mg/kg of body weight) every 2 weeks or standard single-agent systemic therapy (methotrexate, docetaxel, or cetuximab). The primary end point was OS.[6]
- The median OS was 7.5 months (95% CI, 5.5–9.1) in the nivolumab group versus 5.1 months (95% CI, 4.0–6.0) in the standard therapy group. OS was statistically significantly longer with nivolumab than with standard therapy (HRdeath, 0.70; 97.73% CI, 0.51–0.96; P = .01). The estimated 1-year survival rate was approximately 19% higher in patients who received nivolumab (36.0%) than in those who received standard therapy (16.6%).[6][Level of evidence A1]
- There was no statistically significant difference in median PFS between treatment groups. The 6-month PFS rate was 19.7% with nivolumab versus 9.9% with standard therapy.
- The response rate was 13.3% in the nivolumab group versus 5.8% in the standard therapy group.
- Grade 3 or 4 treatment-related adverse events occurred in 13.1% of the patients in the nivolumab group compared with 35.1% of the patients in the standard therapy group.
- Quality-of-life outcomes—including physical, role, and social functioning and pain, sensory, and social contact problems—were stable in the nivolumab group but worse in the standard therapy group. These outcomes were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ) Core Module (QLQ-C30) and the Head and Neck Module (QLQ-H&N35).
- In the subgroup of patients with a PD-L1 expression level of 1% or higher, the HRdeath among patients treated with nivolumab versus standard therapy was 0.55 (95% CI, 0.36–0.83). In the subgroup of patients with a PD-L1 expression level lower than 1%, the HR was 0.89 (95% CI, 0.54–1.45; P = .17 for interaction).
- A randomized, phase III, superiority study in India evaluated the dose of immune checkpoint inhibitors in the setting of palliative care for patients with advanced head and neck cancer. Low-dose IV nivolumab (20 mg every 3 weeks) was added to a triple metronomic chemotherapy regimen of oral methotrexate (9 mg/m2 once weekly), celecoxib (200 mg twice daily), and erlotinib (150 mg once daily). Notably, this nivolumab dose is less than 10% of the dose recommended by the U.S. Food and Drug Administration and the European Medicines Agency. A total of 151 patients were randomly assigned to receive either triple metronomic chemotherapy alone (n = 75) or triple metronomic chemotherapy with nivolumab (n = 76). The primary end point was 1-year OS.[17]
- The addition of low-dose nivolumab to triple metronomic chemotherapy improved the 1-year OS rate from 16.3% (95% CI, 8.0%–27.4%) to 43.4% (95% CI, 30.8%–55.3%) (HR, 0.545; 95% CI, 0.362–0.820; P = .0036).[17][Level of evidence A1]
- The median OS was 6.7 months (95% CI, 5.8–8.1) for patients who received triple metronomic chemotherapy alone and 10.1 months (95% CI, 7.4–12.6) for patients who received triple metronomic chemotherapy with nivolumab (P = .0052).
- The rate of grade 3 or higher adverse events was 50% for patients who received triple metronomic chemotherapy alone and 46.1% for patients who received triple metronomic chemotherapy with nivolumab (P = .744).
Although the control arm in this study cannot be considered standard care, lower doses of immunotherapy appeared to have some benefit in this setting.[18]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Wong LY, Wei WI, Lam LK, et al.: Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck 25 (11): 953-9, 2003. [PUBMED Abstract]
- Vikram B, Strong EW, Shah JP, et al.: Intraoperative radiotherapy in patients with recurrent head and neck cancer. Am J Surg 150 (4): 485-7, 1985. [PUBMED Abstract]
- Spencer SA, Harris J, Wheeler RH, et al.: RTOG 96-10: reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys 51 (5): 1299-304, 2001. [PUBMED Abstract]
- Vargo JA, Ferris RL, Ohr J, et al.: A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 91 (3): 480-8, 2015. [PUBMED Abstract]
- Cohen EEW, Soulières D, Le Tourneau C, et al.: Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393 (10167): 156-167, 2019. [PUBMED Abstract]
- Ferris RL, Blumenschein G, Fayette J, et al.: Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375 (19): 1856-1867, 2016. [PUBMED Abstract]
- Burtness B, Harrington KJ, Greil R, et al.: Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394 (10212): 1915-1928, 2019. [PUBMED Abstract]
- Harrington KJ, Burtness B, Greil R, et al.: Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J Clin Oncol 41 (4): 790-802, 2023. [PUBMED Abstract]
- Tortochaux J, Tao Y, Tournay E, et al.: Randomized phase III trial (GORTEC 98-03) comparing re-irradiation plus chemotherapy versus methotrexate in patients with recurrent or a second primary head and neck squamous cell carcinoma, treated with a palliative intent. Radiother Oncol 100 (1): 70-5, 2011. [PUBMED Abstract]
- Vermorken JB, Mesia R, Rivera F, et al.: Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359 (11): 1116-27, 2008. [PUBMED Abstract]
- Mesía R, Rivera F, Kawecki A, et al.: Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 21 (10): 1967-73, 2010. [PUBMED Abstract]
- Licitra L, Mesia R, Rivera F, et al.: Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol 22 (5): 1078-87, 2011. [PUBMED Abstract]
- Machiels JP, Haddad RI, Fayette J, et al.: Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol 16 (5): 583-94, 2015. [PUBMED Abstract]
- Haddad RI, Harrington K, Tahara M, et al.: Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J Clin Oncol 41 (12): 2166-2180, 2023. [PUBMED Abstract]
- Burtness B: First-Line Nivolumab Plus Ipilimumab in Recurrent/Metastatic Head and Neck Cancer-What Happened? J Clin Oncol 41 (12): 2134-2137, 2023. [PUBMED Abstract]
- Harrington KJ, Ferris RL, Gillison M, et al.: Efficacy and Safety of Nivolumab Plus Ipilimumab vs Nivolumab Alone for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: The Phase 2 CheckMate 714 Randomized Clinical Trial. JAMA Oncol 9 (6): 779-789, 2023. [PUBMED Abstract]
- Patil VM, Noronha V, Menon N, et al.: Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study. J Clin Oncol 41 (2): 222-232, 2023. [PUBMED Abstract]
- Mitchell AP, Goldstein DA: Cost Savings and Increased Access With Ultra-Low-Dose Immunotherapy. J Clin Oncol 41 (2): 170-172, 2023. [PUBMED Abstract]
Latest Updates to This Summary (05/14/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult oropharyngeal cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Oropharyngeal Cancer Treatment are:
- Andrea Bonetti, MD (Pederzoli Hospital)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Oropharyngeal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/head-and-neck/hp/adult/oropharyngeal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389168 ]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.