Technology Transfer Center
NCI has a long history of partnering with private-sector life sciences companies. Often these collaborations support the development of new, innovative technologies or potential new therapies, including those for rare cancers, for which the private sector traditionally has been reluctant to allocate sufficient resources.
Staff from NCI’s Technology Transfer Center work closely with NIH investigators to negotiate agreements with outside parties, including universities and pharmaceutical and biotechnology companies, to facilitate commercialization efforts to benefit public health.
One of the most enduring and successful mechanisms for promoting collaboration between NCI and the private sector is through Cooperative Research and Development Agreements (CRADAs), a mechanism used throughout the federal government. Under a CRADA, NCI researchers and scientists from the partner organization conduct laboratory and early human studies needed to advance promising new technologies or therapeutics to late-stage studies.
CRADAs between NCI and private-sector companies are increasingly used to develop targeted therapies and immunotherapy treatments for both pediatric and adult cancers. For example, through a CRADA with one small biotechnology company, NCI is leading the first trial of an immune-based treatment that will include patients with a rare cancer, called chordoma, for which there are few effective treatments.
The evolution of cancer care toward precision medicine, in which treatments are based on the molecular makeup of tumors rather than on their anatomic location in the body, will require complex clinical trial designs. NCI has developed partnerships with pharmaceutical and biotechnology companies to launch a series of precision medicine trials, some of which, like the MATCH trial, use both FDA-approved and investigational therapies from multiple companies—something that traditionally has been extremely difficult to do.
Small Business Innovation Research and Small Business Technology Transfer
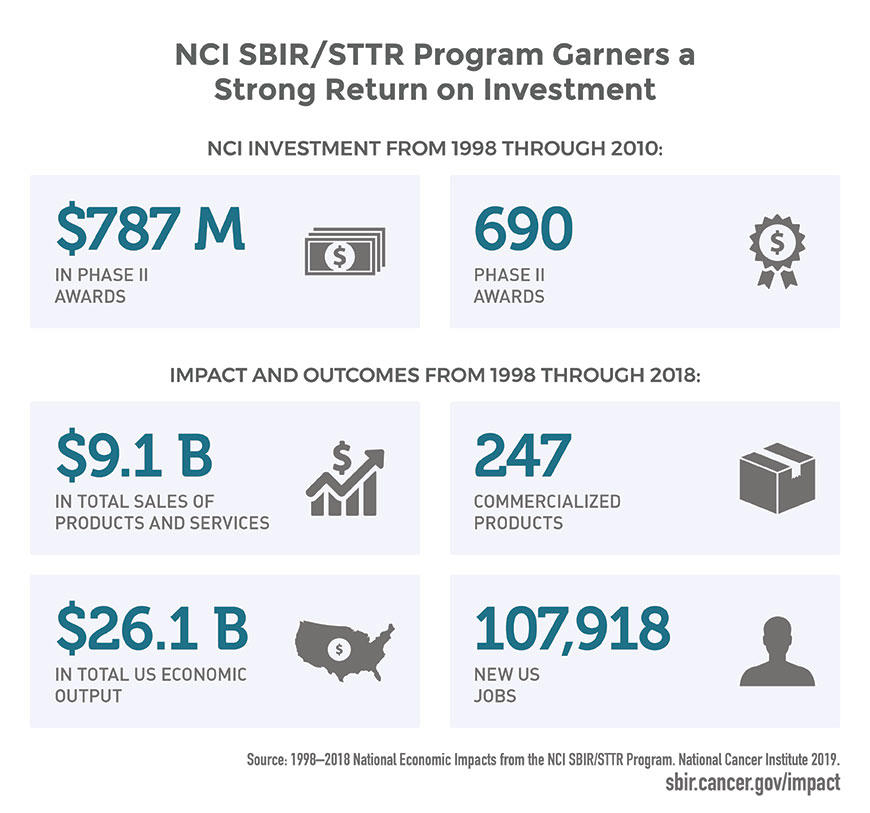
Through its Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs, NCI provides research and development support to small companies at strategic times during the development life cycle of novel cancer-related biomedical products. These funds encourage small, innovative companies to participate in research and development activities that have the potential for private-sector commercialization and public benefit.
The programs are specifically set up to help small businesses develop and commercialize innovative devices, diagnostics, algorithms, and therapeutics to improve cancer research, prevention, detection, and care. These SBIR/STTR awards are often leveraged by small businesses to attract funding from larger firms and venture capital companies.
NCI’s SBIR/STTR programs have supported the development of numerous technologies since 1982. In 2018, the portfolio contained 475 projects, including drugs, in vitro diagnostics, devices, and other technologies.
For example, SBIR supported the development of CYAD-101, a CAR T-cell therapy made from healthy donor immune cells instead of a patient’s own immune cells. CYAD-101 is being tested as a treatment for metastatic colorectal cancer but has the potential to treat other cancers as well. Following the Food and Drug Administration’s (FDA) approval of an investigational new drug application for CYAD-101 in 2018, three phase 1 trials were designed to evaluate the efficacy and optimal dosage of this CAR T-cell therapy against several other cancers that have been challenging to treat, including bladder, pancreatic, ovarian, and triple-negative breast cancer.
A recent economic impact study showed that the SBIR/STTR programs can improve the lives of patients by strengthening the role of small businesses that develop innovative cancer technologies. The study found that NCI received a strong return on investment in supporting small businesses, with an estimate as high as $33 returned for every $1 invested.