FDA Approvals - Cancer Currents Blog
News on recent approvals of cancer therapies by the Food and Drug Administration. Includes expert comments on how the approval will influence patient care and future research.
-
Combination of Immunotherapy Drugs Approved for Metastatic Colorectal Cancer
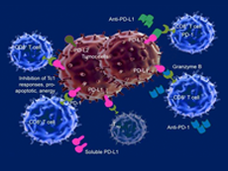
The FDA has approved the combination of the immune checkpoint inhibitors ipilimumab (Yervoy) and nivolumab (Opdivo) for the treatment of patients with metastatic colorectal cancer whose tumor cells have defects that affect their ability to repair DNA.
-
New Immunotherapy Option Approved for Cervical Cancer, Rare Lymphoma
FDA has approved pembrolizumab (Keytruda) for some women with advanced cervical cancer and some patients with primary mediastinal large B-cell lymphoma (PMBCL), a rare type of non-Hodgkin lymphoma.
-
FDA Alters Approved Use of Two Checkpoint Inhibitors for Bladder Cancer
FDA has changed the approved uses of the immunotherapy drugs pembrolizumab (Keytruda) and atezolizumab (Tecentriq) to treat the most common form of bladder cancer. The change is based on whether patients’ tumors have a specific biomarker.
-
Approval Expanded for Venetoclax in Chronic Lymphocytic Leukemia
FDA expanded the approval of venetoclax (Venclexta) for people with chronic lymphocytic leukemia (CLL) to include those whose cancer has progressed after previous treatment, regardless of whether their cancer cells have the deletion 17p gene alteration.
-
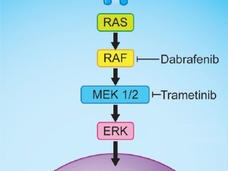
Dabrafenib–Trametinib Combination Approved for Melanoma, Anaplastic Thyroid Cancer
FDA recently approved the targeted-drug combination to treat patients with advanced melanoma and a subset of patients with a rare and aggressive form of thyroid cancer whose tumors have a specific mutation in the BRAF gene.
-
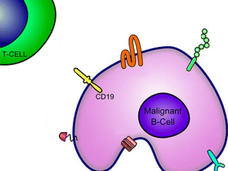
FDA Approves Second CAR T-Cell Therapy for Lymphoma
FDA has approved tisagenlecleucel (Kymriah) for certain kinds of non-Hodgkin lymphoma. Read about the trial that led to the approval and what the approval means for people with lymphoma.
-
FDA Approves Nivolumab and Ipilimumab Combination for Advanced Kidney Cancer
FDA has approved the combination of two immunotherapy drugs, nivolumab (Opdivo) and ipilimumab (Yervoy), as an initial treatment for some patients with advanced kidney cancer. Learn how this approval will affect patient care.
-
Rucaparib Approved as Maintenance Treatment for Some Recurrent Ovarian Cancers
FDA has expanded its approval of rucaparib (Rubraca) as a maintenance therapy for women with recurrent ovarian, fallopian tube, or primary peritoneal cancer whose tumors shrank after subsequent treatment with a platinum-based chemotherapy.
-
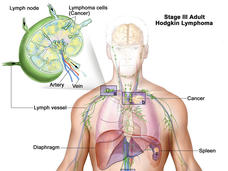
Brentuximab Approved for Initial Treatment of Advanced Hodgkin Lymphoma
The FDA has expanded the approved uses of brentuximab (Adcetris) in people with Hodgkin lymphoma. Under the new approval, brentuximab can be used in combination with three other chemotherapy drugs as an initial treatment in patients with advanced disease.
-
Abemaciclib Approval Expands Initial Treatment Options for Advanced Breast Cancer
FDA has approved the CDK4/6 inhibitor abemaciclib (Verzenio) as a first-line treatment in some women with advanced or metastatic breast cancer. Under the approval, the drug must be used in combination with an aromatase inhibitor.
-
FDA Approves Apalutamide for Some Men with Prostate Cancer
In the trial that led to the approval, apalutamide (Erleada) delayed cancer metastasis for men with prostate cancer that is resistant to androgen deprivation therapy.
-
Cabozantinib Approval Expands Initial Treatment Options for Advanced Kidney Cancer
The Food and Drug Administration has approved cabozantinib (Cabometyx®) as an initial treatment for patients with advanced renal cell carcinoma, the most common type of kidney cancer.
-
Abiraterone Approved for Earlier Use in Men with Metastatic Prostate Cancer
The Food and Drug Administration (FDA) has expanded the approval of abiraterone (Zytiga®) for men with prostate cancer. The agency approved abiraterone, in combination with the steroid prednisone, for men with metastatic prostate cancer that is responsive to hormone-blocking treatments (also known as castration-sensitive) and is at high risk of progressing.
-
FDA Approves New Treatment for Certain Neuroendocrine Tumors
People with cancerous neuroendocrine tumors (NETs) that affect the digestive tract now have a new treatment option. On January 29, FDA approved the targeted treatment lutetium Lu 177 dotatate (Lutathera®) for adult patients with advanced NETs that affect the pancreas or gastrointestinal tract.
-
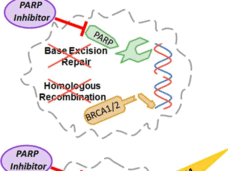
Olaparib Approved for Treating Some Breast Cancers with BRCA Gene Mutations
The drug olaparib (Lynparza®) is the first treatment approved by the Food and Drug Administration for patients with metastatic breast cancer who have inherited mutations in the BRCA1 or BRCA2 genes.
-
Nilotinib Can Be Discontinued in Some Patients with Chronic Myelogenous Leukemia
On December 22, FDA approved an update to the label of nilotinib (Tasignia) that states that some patients with CML who are taking nilotinib and whose cancer has been in remission for an extended period can safely stop taking it.
-
Genomic Profiling Tests Cleared by FDA Can Help Guide Cancer Treatment, Clinical Trial Enrollment
The FDA has recently approved two tests to identify genetic alterations in tumors. One of the tests can be used to identify patients who may be candidates to receive specific targeted therapies.
-
Brentuximab Vedotin Approved for Two Rare Lymphomas
FDA has approved brentuximab vedotin (Adcetris®) for the treatment of adults who have been treated previously for either primary cutaneous anaplastic large cell lymphoma or CD30-expressing mycosis fungoides, two rare lymphomas that start as rashes on the skin.
-
Acalabrutinib Receives FDA Approval for Mantle Cell Lymphoma
The FDA has granted accelerated approval to acalabrutinib (Calquence®) for the treatment of adults with mantle cell lymphoma whose cancer has progressed after receiving at least one prior therapy.
-
FDA Approves Alectinib For Initial Treatment of ALK-Positive Lung Cancer
FDA has approved alectinib (Alecensa) as a first-line treatment option for patients with advanced non-small cell lung cancer that is ALK positive. Alectinib is the third ALK inhibitor to be approved in this setting.