FDA Approvals - Cancer Currents Blog
News on recent approvals of cancer therapies by the Food and Drug Administration. Includes expert comments on how the approval will influence patient care and future research.
-
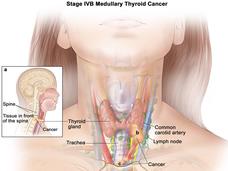
Selpercatinib Approved for Thyroid and Lung Cancers with RET Gene Alterations
FDA has granted accelerated approval for selpercatinib (Retevmo) to treat certain patients with thyroid cancer or non-small cell lung cancer whose tumors have RET gene alterations. The drug, which works by blocking the activity of RET proteins, was approved based on the results of the LIBRETTO-001 trial.
-
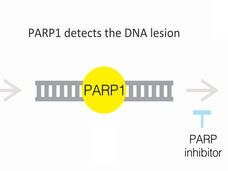
With Two FDA Approvals, Prostate Cancer Treatment Enters the PARP Era
FDA has approved olaparib (Lynparza) and rucaparib (Rubraca) to treat some men with metastatic prostate cancer. The PARP inhibitors are approved for men whose cancers have stopped responding to hormone treatment and have specific genetic alterations.
-
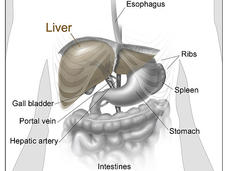
Atezolizumab Plus Bevacizumab Approved to Treat Liver Cancer
FDA has approved atezolizumab (Tecentriq) plus bevacizumab (Avastin) as an initial treatment for some people with advanced liver cancer. This is the first approval in 13 years for a treatment that is more effective than the current standard, sorafenib.
-
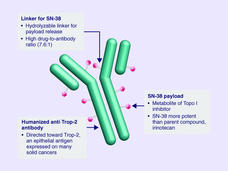
Sacituzumab Govitecan Approved for Metastatic Triple-Negative Breast Cancer
FDA has approved sacituzumab govitecan (Trodelvy) for the treatment of triple-negative breast cancer that has spread to other parts of the body. Under the approval, patients must have already undergone at least two prior treatment regimens.
-
Encorafenib, Cetuximab Combination Approved for Metastatic Colorectal Cancer
The Food and Drug Administration has approved encorafenib (Braftovi) in combination with cetuximab (Erbitux) to treat adults with metastatic colorectal cancer whose tumors have a specific mutation in the BRAF gene, called V600E.
-
Selumetinib Approved by FDA to Treat Children with NF1
The Food and Drug Administration (FDA) has approved selumetinib (Koselugo) to treat children with neurofibromatosis 1 (NF1), a genetic disorder that causes tumors, called plexiform neurofibromas, to form throughout the nervous system.
-
Avapritinib Approved to Treat GIST with a Rare Gene Alteration
Avapritinib (Ayvakit) has been approved for adults with gastrointestinal stromal tumors (GIST) whose tumors have an alteration in a portion of the PDGFRA gene called exon 18. The approval applies to those whose tumors cannot be removed with surgery or have spread.
-
Enfortumab Vedotin Approved for Recurrent Bladder Cancer
Enfortumab vedotin-ejfv (Padcev) has been approved for people with advanced bladder cancer. FDA granted the drug accelerated approval for cancers that have progressed despite previous treatments.
-
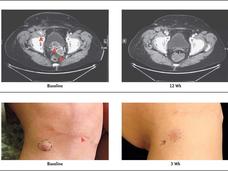
For Metastatic HER2-Positive Breast Cancer, New Treatments Emerge
Tucatinib improved survival for women in the HER2CLIMB trial, including some whose cancer had spread to the brain. Trastuzumab deruxtecan improved survival and shrank many tumors in the DESTINY-Breast01 trial, which led to its accelerated approval.
-
FDA Approves Entrectinib Based on Tumor Genetics Rather Than Cancer Type
FDA has approved entrectinib (Rozlytrek) for the treatment of children and adults with tumors bearing an NTRK gene fusion. The approval also covers adults with non-small cell lung cancer harboring a ROS1 gene fusion.
-
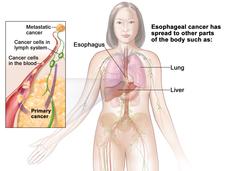
Pembrolizumab Approved for Some Patients with Advanced Esophageal Cancer
FDA has approved the immunotherapy drug pembrolizumab (Keytruda) to treat some patients with advanced esophageal cancer. Patients must have certain levels of the protein PD-L1 on their tumors, as determined by an FDA-approved test.
-
Combination Therapy with Venetoclax Approved for Chronic Lymphocytic Leukemia
The Food and Drug Administration has approved venetoclax (Venclexta) in combination with obinutuzumab (Gazyva) for the initial treatment of adults with chronic lymphocytic leukemia or small lymphocytic lymphoma.
-
T-DM1 Approval Expanded to Include Some Women with Early-Stage HER2-Positive Breast Cancer
FDA has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla), also called T-DM1, to include adjuvant treatment in some women with early-stage HER2-positive breast cancer.
-
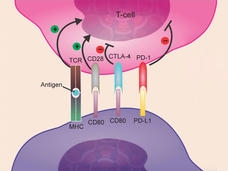
Atezolizumab Approved for Some Patients with Triple-Negative Breast Cancer
FDA has approved atezolizumab (Tecentriq) in combination with chemotherapy for the treatment of some women with advanced triple-negative breast cancer. This is the first FDA-approved regimen for breast cancer to include immunotherapy.
-
Pembrolizumab Now Second Immunotherapy Approved to Treat Merkel Cell Carcinoma
FDA has approved pembrolizumab (Keytruda) to treat people with Merkel cell carcinoma, a rare and deadly form of skin cancer. The approval covers use of the drug to treat locally advanced or metastatic forms of the disease.
-
Atezolizumab Approved for Initial Treatment of Metastatic Lung Cancer
On December 6, 2018, the Food and Drug Administration (FDA) approved atezolizumab (Tecentriq) in combination with a standard three-drug regimen as an initial treatment for advanced lung cancer that does not have EGFR or ALK mutations.
-
FDA Approvals Bring New Options for Older Patients with AML
FDA has approved venetoclax (Venclexta) and glasdegib (Daurismo) for use in people with acute myeloid leukemia aged 75 and older and those with health conditions that prevent them from receiving the intensive chemotherapy regimen that is the standard initial treatment.
-
Immunotherapy Drug Cemiplimab Approved for Advanced Squamous Cell Skin Cancer
The Food and Drug Administration approved the immunotherapy drug cemiplimab (Libtayo) for an advanced form of cutaneous squamous cell carcinoma (SCC), a common type of skin cancer. It is the first agent to be approved specifically for advanced SCC.
-
Moxetumomab Approved by FDA for Hairy Cell Leukemia
The FDA has approved moxetumomab pasudotox (Lumoxiti), a bacterial toxin–based drug, for the treatment of some patients with hairy cell leukemia (HCL). Moxetumomab is approved to treat patients with HCL who have already undergone at least two lines of standard treatments.
-
Ribociclib Approval Expanded for Some Women with Advanced Breast Cancer
FDA has expanded the approved uses of ribociclib (Kisqali) for women with advanced breast cancer, including new uses in pre- and postmenopausal women. It’s the first approval under a new FDA program to speed the review of cancer drugs.