FDA Approvals - Cancer Currents Blog
News on recent approvals of cancer therapies by the Food and Drug Administration. Includes expert comments on how the approval will influence patient care and future research.
-
Trial Suggests Expanded Role for Blinatumomab in Treating ALL
The immunotherapy drug blinatumomab (Blincyto) extends life for people with acute lymphoblastic leukemia who are in remission, even those with no signs of disease after initial treatment, a trial has found.
-
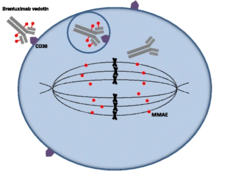
Brentuximab Approved for High-Risk Hodgkin Lymphoma in Children and Adolescents
Based on an NCI-sponsored clinical trial conducted by the Children’s Oncology Group, FDA has approved the drug brentuximab vedotin (Adcetris) in combination with chemotherapy for some children and adolescents with Hodgkin lymphoma.
-
Sodium Thiosulfate Approved to Reduce Chemo-Related Hearing Loss in Children with Cancer
The chemotherapy cisplatin often causes permanent hearing loss. Sodium thiosulfate (Pedmark) is the first treatment approved by FDA that can reduce the risk of hearing loss and the severity of damage to the inner ear in children treated with cisplatin.
-
Enhertu Marks First Targeted Therapy for HER2-Mutant Lung Cancer
On August 11, the Food and Drug Administration (FDA) gave accelerated approval to trastuzumab deruxtecan (Enhertu) for adults with non-small cell lung cancer (NSCLC) that has a specific mutation in the HER2 gene. Around 3% of people with NSCLC have this kind of HER2 mutation.
-
Dabrafenib–Trametinib Combination Approved for Solid Tumors with BRAF Mutations
FDA has approved the combination of the targeted drugs dabrafenib (Tafinlar) and trametinib (Mekinist) for nearly any type of advanced solid tumor with a specific mutation in the BRAF gene. Data from the NCI-MATCH trial informed the approval.
-
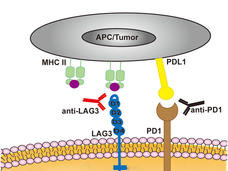
Opdualag Becomes First FDA-Approved Immunotherapy to Target LAG-3
The immunotherapy treatment, which combines the LAG-3 inhibitor relatlimab and PD-1 inhibitor nivolumab, becomes the first new immune checkpoint inhibitor approved in 8 years. Both drugs are given to patients via a single infusion to treat advanced melanoma.
-
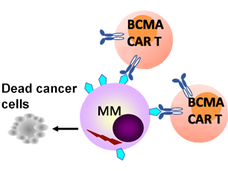
Carvykti Approval Marks Second CAR T-Cell Therapy for Multiple Myeloma
People with advanced multiple myeloma now have another option for CAR T-cell therapy with the recent approval of ciltacabtagene autoleucel (Carvykti). Like the first approved CAR T-cell therapy, Carvykti targets the BCMA protein on myeloma cells.
-
Tecartus Becomes First CAR T-Cell Therapy Approved for Adults with ALL
The CAR T-cell therapy Tecartus has become the first such treatment approved by FDA to treat adults with acute lymphoblastic leukemia (ALL). The approval is for patients whose cancer has not responded to treatment or returned after treatment.
-
Adjuvant Immunotherapy Approved for Some Patients with Lung Cancer
Atezolizumab (Tecentriq) is now the first immunotherapy approved by FDA for use as an additional, or adjuvant, treatment for some patients with non-small cell lung cancer. The approval was based on results of a clinical trial called IMpower010.
-
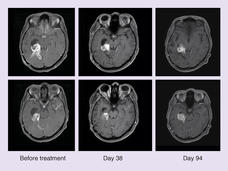
Belzutifan Approved to Treat Tumors Linked to Inherited Disorder VHL
FDA has approved belzutifan (Welireg) to treat adults with von Hippel-Lindau disease (VHL) who have tumors of the kidney, brain, nervous system, or pancreas. The drug may help these patients avoid or delay surgery by shrinking their tumors.
-
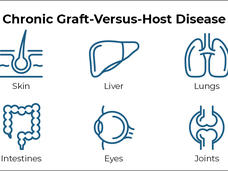
FDA Approves Belumosudil to Treat Chronic Graft-Versus-Host Disease
FDA has approved belumosudil (Rezurock) for the treatment of chronic graft-versus-host disease (GVHD). The approval covers the use of belumosudil for people 12 years and older who have already tried at least two other therapies.
-
FDA Approval of Rylaze Will Address Drug Shortage for Childhood ALL
FDA has approved a new form of asparaginase called Rylaze. The drug was developed to help alleviate shortages of Erwinia asparaginase, a key part of treatment for children and adults with acute lymphoblastic leukemia.
-
FDA Approval of KRAS Inhibitor Sotorasib for Lung Cancer Hailed as Milestone
FDA has approved the first KRAS-blocking drug, sotorasib (Lumakras). The approval, which covers the use of sotorasib to treat some patients with advanced lung cancer, sets the stage for other KRAS inhibitors already in development, researchers said.
-
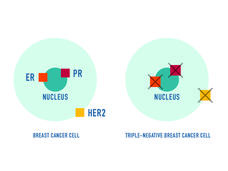
Sacituzumab Govitecan Earns Full Approval for Triple-Negative Breast Cancer
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC). The update follows last year’s accelerated approval of the drug for people with TNBC.
-
FDA Approves BCMA-Targeted CAR T-Cell Therapy for Multiple Myeloma
The Food and Drug Administration has approved idecabtagene vicleucel (Abecma) for some people with multiple myeloma. The approval is based, in part, on a small study in which ide-cel partially or completely shrank tumors in 72% of patients.
-
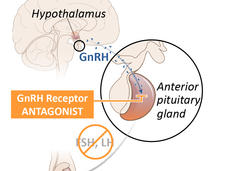
Relugolix Approval Expected to Alter Treatment for Advanced Prostate Cancer
FDA’s recent approval of relugolix (Orgovyx) is expected to affect the treatment of men with advanced prostate cancer. A large clinical trial showed that relugolix was more effective at reducing testosterone levels than another common treatment.
-
Cancer “Liquid Biopsy” Blood Test Gets Expanded FDA Approval
FDA has expanded the approved uses of the FoundationOne Liquid CDx blood test, known as a liquid biopsy, that can help doctors pick specific treatments for some people with cancer. When used in this way, the test is known as a companion diagnostic.
-
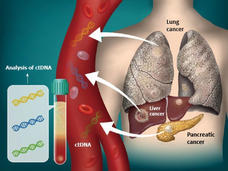
FDA Approves Blood Tests That Can Help Guide Cancer Treatment
FDA has recently approved two blood tests, known as liquid biopsies, that gather genetic information to help inform treatment decisions for people with cancer. This Cancer Currents story explores how the tests are used and who can get the tests.
-
CAR T-Cell Therapy Approved by FDA for Mantle Cell Lymphoma
A CAR T-cell therapy called brexucabtagene autoleucel (Tecartus) has been approved by the Food and Drug Administration (FDA) for some patients with mantle cell lymphoma. This is the third CAR T-cell therapy approved by FDA for patients with cancer.
-
A New FDA Approval Furthers the Role of Genomics in Cancer Care
FDA’s approval of pembrolizumab (Keytruda) to treat people whose cancer is tumor mutational burden-high highlights the importance of genomic testing to guide treatment, including for children with cancer, according to NCI Director Dr. Ned Sharpless.