November 2019 - Cancer Currents Blog
-
Gilteritinib Improves Survival in AML with FLT3 Mutations
People with relapsed or refractory acute myeloid leukemia (AML) with FLT3 gene mutations treated with gilteritinib had improved survival, higher rates of remission, and fewer side effects than those treated with chemotherapy, a recent trial found.
-
Children with Acute Lymphoblastic Leukemia Can Skip Radiation to the Brain
Only 1.5% of children with acute lymphoblastic leukemia who skipped radiation had a recurrence in the central nervous system, according to a recent trial. The therapy, which is intended to prevent such a recurrence, can have devastating side effects.
-
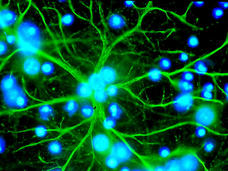
Some Brain Cells May Help to Fuel Cancer Metastasis
Brain cells called astrocytes can activate PPAR-gamma, a growth protein in cancer cells that helps them gain a foothold in the brain, a new study shows. The findings suggest that drugs that block PPAR-gamma activity may help treat brain metastases.
-
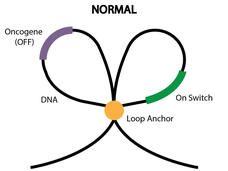
Epigenetic Changes Pinpointed as the Cause of Some GISTs
Scientists may have pinpointed the cause of some gastrointestinal stromal tumors (GISTs), a rare cancer, according to a new NCI-funded study. However, the culprit isn’t a harmful genetic mutation, but another type of genetic change, what are called epigenetic alterations.
-
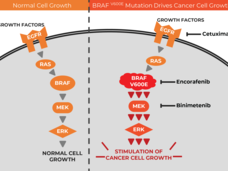
Targeted Drug Trio Improves Survival in Colorectal Cancer with BRAF Mutations
For people with colorectal cancer with a specific mutation in the BRAF gene, a treatment regimen of three targeted drugs can improve how long they live without increasing their risk of serious side effects, results from a new clinical trial show.
-
Prescribing Exercise as Cancer Treatment: A Conversation with Dr. Kathryn Schmitz
Updated guidelines on exercise for those living with cancer and cancer survivors were recently released. In this conversation, Dr. Kathryn Schmitz discusses what these new guidelines mean for doctors, patients, and survivors.
-
Home Is Where the Heart Is
Dr. Ned Sharpless discusses his return as NCI director after serving as acting commissioner of the Food and Drug Administration for 7 months.