August 2019 - Cancer Currents Blog
-
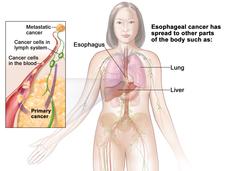
Pembrolizumab Approved for Some Patients with Advanced Esophageal Cancer
FDA has approved the immunotherapy drug pembrolizumab (Keytruda) to treat some patients with advanced esophageal cancer. Patients must have certain levels of the protein PD-L1 on their tumors, as determined by an FDA-approved test.
-
Study Tests Immunotherapy in People with Cancer and Autoimmune Diseases
An NCI-funded clinical trial is testing the immunotherapy drug nivolumab (Opdivo) in people who have advanced cancer and an autoimmune disease, such as rheumatoid arthritis, lupus, or multiple sclerosis, who are often excluded from such trials.
-
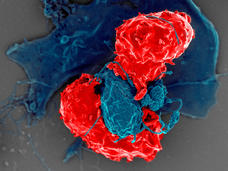
Newly Discovered ‘Don’t Eat Me’ Signal May be a Target for Cancer Immunotherapy
Researchers have identified a protein called CD24 that may be a new target for cancer immunotherapy. The protein is a ‘don’t eat me’ signal that prevents immune cells called macrophages from engulfing and eating cells.
-
The Childhood Cancer Data Initiative: Transforming the Pediatric Cancer Landscape through Sharing Data
To prepare for the proposed Childhood Cancer Data Initiative, NCI sponsored a 3-day symposium that brought together pediatric cancer researchers, advocates, and other stakeholders.
-
MGUS to Myeloma: Study Suggests Risk of Progression Can Change
A person’s risk of progressing from a benign condition called monoclonal gammopathy of undetermined significance (MGUS) to the blood cancer multiple myeloma can change over time, according to a new study.
-
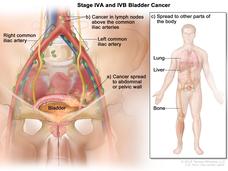
Targeted Drug Erdafitinib Benefits Some Patients with Advanced Bladder Cancer
New clinical trial findings confirm that the targeted therapy erdafitinib (Balversa) can benefit patients with advanced bladder cancer whose tumors have a genetic alteration in one of the four FGFR genes.