Manipulating an Immune Cell May Make Radiation Therapy More Effective, Study Suggests
, by Sharon Reynolds
Immunotherapy drugs to treat cancer are often most effective when used alongside other treatments, including chemotherapy and targeted therapies. But researchers are still trying to figure out how best to use one of the most common cancer treatments, radiation, to help make immunotherapy more effective.
A team of researchers has now investigated the other side of the coin, that is, whether there’s a way to manipulate the immune system to make radiation therapy a more potent tool against cancer. The findings from their study suggest that there may be.
Led by researchers from the University of Chicago, the study zeroed in on immune cells called myeloid-derived suppressor cells (MDSCs). Their day-to-day job is to be a safety officer of sorts, directing T cells and other cell-killing immune cells to keep their distance from what those cells perceive as threats: “Go away,” they tell the killer immune cells. “Your services aren’t needed here.”
In cancer, however, tumor cells take advantage of MDSCs, using them as a way to escape attack by the immune system’s most lethal killers, including T cells. In this new study, researchers discovered why this appears to happen. The key player: a protein called BAMBI.
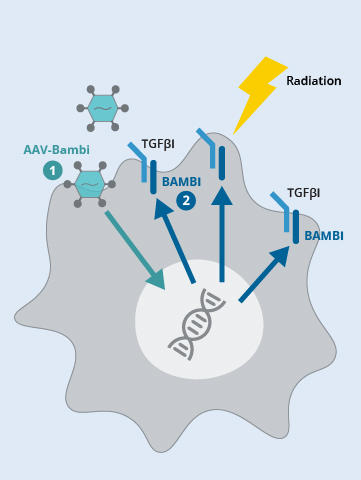
In experiments in mice, the research team, led by Liangliang Wang, Ph.D., showed that after radiation to a tumor, levels of BAMBI plummeted in MDSCs. With BAMBI in short supply, more MDSCs flowed into tumors, and they became much more efficient at driving T cells away from tumors.
But, according to findings published December 15, 2023, in the Journal of Clinical Investigation, they also showed that when BAMBI levels were increased in MDSCs—which the researchers achieved with the help of gene therapy—T cells flooded into the tumor after the radiation treatment.
And the T cells didn’t stop there: They went on to seek out and kill tumors elsewhere in the body.
Radiation therapy continues to be an important form of cancer treatment, and any way of making it more effective would be a major advance, said the study’s senior researcher, Ralph Weichselbaum, M.D., of the University of Chicago Medicine Comprehensive Cancer Center.
However, delivering BAMBI to cells is no easy task, and a lot has to be learned before human studies of a BAMBI-delivering treatment combined with radiation can be launched, he added.
Many contributors to radiation resistance
Radiation therapy, one of the oldest forms of cancer treatment, damages the DNA inside cancer cells, leading to their death days or weeks after treatment.
But cancer cells that can rapidly repair their DNA may quickly bounce back after radiation therapy. “For a long time, it was thought that the entire effect of radiation was based on DNA damage in the tumor,” said Dr. Weichselbaum.
Researchers now understand that other types of cells that share space in tumors with cancer cells, including so-called suppressive immune cells that tamp down the immune response to perceived threats, can also be affected by radiation. One consequence can be a reduction in the ability of immune cells to kill cancer cells.
Immunosuppressive immune cells exist for a very good reason, explained Pataje Prasanna, Ph.D., from NCI's Radiation Research Program, who was not involved with the new study. MDSCs, for example, play an important role in reducing inflammation and preventing autoimmune diseases.
“The body needs to maintain a balance between pro-inflammatory and anti-inflammatory states,” Dr. Prasanna said. “Some inflammation is necessary, but not too much. That's why you need immunosuppression. But cancer cells can [exploit] this.”
Understanding how other cells within and around tumors—and the molecules they produce—help cancer cells survive after radiation therapy is important, explained Hua Laura Liang, Ph.D., who helped lead the new study, because it can point to potential ways to make this commonly used treatment more effective.
It’s very likely that there are many contributors to radiation therapy resistance beyond those directly linked to cancer cells, Dr. Weichselbaum said. And those same contributors may also influence how best to combine radiation therapy with immune-based treatments. To date, human studies of such combinations have had mixed results, he continued.
With their new study, he said, they wanted to know: “What other cells might you have to amplify or eliminate to get [the combination] to work?”
A cascade to boost tumor cell survival after radiation therapy
A protein called TGF-β is known to play a direct role in resistance to radiation and other cancer treatments caused by suppressive immune cells like MDSCs. But to date, and to the frustration of researchers, efforts to directly manipulate TGF-β in people have proven unsuccessful.
So, over the years, the focus has switched to understanding how TGF-β works in specific cell types, and whether there are ways to indirectly affect its behavior.
A team from Dr. Weichselbaum’s laboratory, led by Dr. Wang, has been working to better understand how TGF-β works in MDSCs. Previous studies from their team and others have indicated that TGF-β’s influence on MDSCs’ behavior is likely related to a complex chain of molecular events that involves BAMBI.
So the researchers decided it was time to dig deeper into precisely what BAMBI does in MDSCs.
They began by examining BAMBI levels in tumor samples of people with four different types of cancer who’d had radiation therapy. People whose tumors had higher amounts of BAMBI lived longer overall, they found, and their tumors had more cancer cell–killing T cells and fewer MDSCs than those whose tumors had lower amounts of BAMBI.
Further work in mice found that BAMBI doesn’t appear to be coming from cancer cells, but from MDSCs in and around the tumor.
In additional experiments, they found that radiation treatment disrupted the genetic instructions MDSCs need to make BAMBI. The radiation-induced free fall in BAMBI production had numerous consequences, including for critical cell functions driven by TGF-β. In mice, for example, following radiation, BAMBI-deficient MDSCs flooded into tumors.
By contrast, in MDSCs with larger amounts of BAMBI, TGF-β’s influence was greatly diminished, and these cells stayed out of the tumor microenvironment, leaving cancer cells more vulnerable to radiation.
Bringing back BAMBI with gene therapy
Most often, targeted cancer therapies work by disabling one or more molecules that are overproduced in tumor cells and that are fueling tumor growth. But with BAMBI, there’s not too much of it in MDSCs—there’s too little.
So, Dr. Wang and his colleagues wondered, would it be possible to boost BAMBI levels? And if so, would that keep the MDSCs away from tumors after radiation therapy?
To answer the question, they used a harmless virus to deliver the genetic instructions for producing BAMBI into MDSCs.
When the researchers injected this BAMBI-delivery vehicle directly into tumors in mice, tumor growth slowed. When they combined this strategy with radiation therapy, the effect on tumors was even greater, and much better than in tumors treated with radiation therapy alone. MDSCs isolated from the tumors that received both treatments showed a reduced ability to suppress other immune cells and nearby T cells had improved abilities to kill cancer cells.
The team saw even more striking results in two mouse models of metastatic cancer. In one model, in mice that spontaneously develop metastatic lung cancer, the combination shrank the primary lung tumors as well as metastases elsewhere in the body.
And in mice with metastases from melanoma, a deadly type of skin cancer, the combination of the BAMBI-boosting virus, radiation to the primary tumor, and an immunotherapy drug shrank both the primary tumor and metastatic tumors more than the BAMBI-boosting virus and radiation therapy alone.
Driving out MDSCs from human tumors?
Using viruses to deliver genetic instructions to cells is no longer science fiction. It’s now a common strategy in various gene therapies, some of which the Food and Drug Administration (FDA) has approved for the treatment of rare diseases.
Dr. Weichselbaum’s lab is planning to look for a virus that may have—or could be engineered to have—a specific affinity for MDSCs to test in further studies. “In theory, you could inject such a virus directly into a tumor and get a body-wide effect,” he explained.
They’re also starting work with a colleague in the University of Chicago’s Department of Chemistry to identify drugs that might be able to stop the damage to the genetic instructions for BAMBI in MDSCs in the first place.
Future studies could also look at whether manipulating BAMBI can help prevent a side effect of radiation therapy called radiation fibrosis, in which scarred tissue accumulates after treatment, Dr. Prasanna said. “We know TGF-β plays a critical role in that process,” he explained.
“One reason radiotherapy and immunotherapy might not work as well together as they could is radiation-induced immunosuppression,” Dr. Weichselbaum said. “We don’t yet know if suppressing these MDSCs is going to work to change that, but it’s definitely worth trying.”