Oncolytic Virus Enables the Immune System to Attack Tumors
, by Shana Spindler
One of the most important findings in cancer research in the past two decades is that tumors can create force field-like protection against the immune system. A new study has identified a way to break through that force field to improve cancer treatment, by taking advantage of a virus that can specifically infect cancer cells.
Oncolytic viruses, as these cancer cell-infecting viruses are known, thrive inside cancer cells. There, they replicate rapidly, destroying the cells by breaking them open and also exposing the cell’s contents to the immune system. Not only do these viruses naturally disrupt cancer, but they can be modified to carry other genes directly into cancer cells that boost the treatment’s ability to destroy tumors.
One oncolytic virus, an immunotherapy called T-VEC, has been approved by the Food and Drug Administration (FDA) for the treatment of metastatic melanoma. Oncolytic virus therapies for additional types of cancer have yet to be approved, although several are being tested in clinical trials.
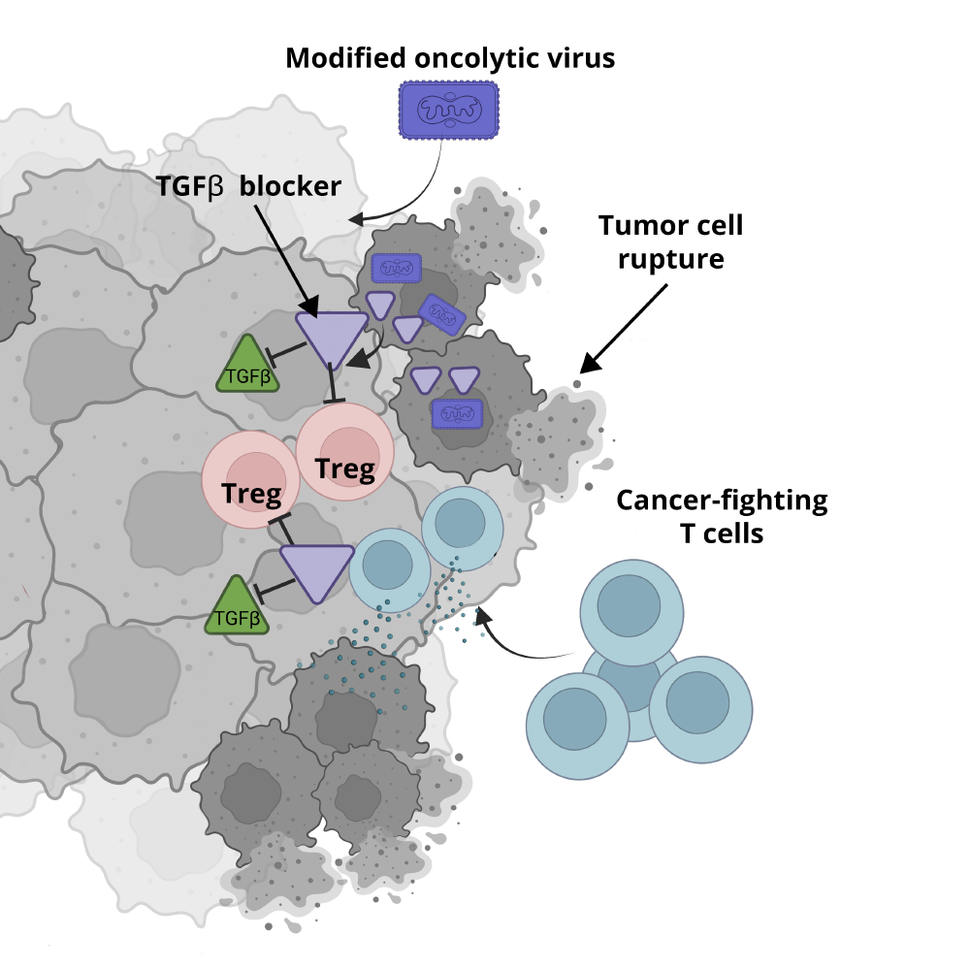
In the new study, funded in part by NCI, Greg Delgoffe, Ph.D., of the University of Pittsburgh School of Medicine, and his colleagues created a modified oncolytic virus that can transport the genetic instructions for a potent cancer treatment directly into cancer cells.
Those instructions cause the cell to produce a protein that blocks the activity of another protein, called TGF-beta, that helps protect cancer cells from being attacked by the immune system.
In mice with head and neck tumors, treatment with the modified virus shrank tumors that could not be affected by the nonmodified oncolytic virus, according to findings published in the October 2 issue of the Journal of Experimental Medicine. The researchers also found that combining the modified oncolytic virus treatment with another type of immunotherapy was even more effective than the oncolytic virus therapy alone.
In addition to demonstrating the potential of using a virus to deliver a drug “payload,” said James Gulley, M.D., Ph.D., co-director of NCI’s Center for Immuno-Oncology, the new study is “another piece of the puzzle showing the importance of TGF-beta” in suppressing the immune response against cancer.
Many experimental drugs block TGF-beta, Dr. Gulley continued, but they often cause serious side effects because they also affect healthy cells. The oncolytic virus developed in this study “is interesting,” he said, because it blocks TGF-beta in tumor cells only, which could reduce side effects from the treatment.
A TGF-beta blocking payload
For more than a century, researchers have known about viruses that can infect and kill cancer cells without harming healthy tissue. Although these oncolytic viruses appear to be safe, and many have shown promising activity against cancer in clinical trials, a few logistical challenges have hindered their widespread use as cancer treatments.
One challenge is the need to inject oncolytic viruses directly into tumors, so that the immune system doesn’t eliminate the virus before it can infect the cancer cells. In addition, specialized resources and training are needed to safely store and administer these viruses to people.
Researchers are working through these issues in hopes of expanding oncolytic virus therapy to more cancers. In the meantime, they are also exploring ways of using oncolytic viruses—through a bit of genetic engineering—to deliver more effective cancer-fighting payloads.
Some oncolytic viruses, for example, have been designed to enhance the cancer-seeking ability of immune cells. In this new study, Dr. Delgoffe’s team took the opposite approach: They designed an oncolytic virus with a payload that would block the signals that tumors cells use to stop immune cells from attacking them.
“This [approach] is brand new in the field,” he said.
The researchers started by trying to identify the most potent immune-suppressing signals in the tumor. To do so, they took advantage of a mouse model of head and neck cancer that was resistant to some forms of immunotherapy. A series of experiments using an unmodified oncolytic virus pointed at TGF-beta as a critical source of that resistance.
That finding wasn’t necessarily surprising. Many other studies have implicated TGF-beta in fueling cancer. And researchers have tried to find drugs that block the activity of TGF-beta in tumors for decades, Dr. Delgoffe explained. Because the protein also plays an important role in maintaining normal cell functions, researchers have sought ways to target TGF-beta only in cancer cells, he said.
To this end, Dr. Delgoffe teamed up with Andrew Hinck, Ph.D., a University of Pittsburgh structural biologist who had designed a protein that could block TGF-beta activity. They inserted the genetic instructions for the TGF-beta–blocking protein into an oncolytic virus for targeted delivery into tumor cells.
Next, the team injected the modified oncolytic virus directly into tumors of mice with oncolytic virus-resistant head and neck cancer. Over about 2 months, the tumors shrank, disappearing completely in about half of the mice. In addition, the treatment appeared to have no obvious side effects.
This last finding is encouraging because experimental therapies that target TGF-beta in all cells throughout the body have had concerning side effects in previous clinical trials, Dr. Gulley explained, including abnormal bleeding.
Combining the oncolytic virus with checkpoint inhibitors
Next, the team tested the modified oncolytic virus in a different, more aggressive cancer: a mouse model of melanoma that is resistant to immunotherapy drugs called immune checkpoint inhibitors.
These drugs, which include nivolumab (Opdivo) and pembrolizumab (Keytruda), are already standard treatments for melanoma. Although the drugs can be highly effective for some patients, resistance often develops.
After injection of a single dose of the modified oncolytic virus alone, tumors disappeared in 20% of the mice. But when the mice received an immune checkpoint inhibitor after the oncolytic virus treatment, tumors disappeared in more than two-thirds of mice.
By contrast, all the mice that received the checkpoint inhibitor or an unmodified oncolytic virus alone died from their disease within a month.
To use the immune system to kill cancer, as is the case with immune checkpoint inhibitors, you need to block immune-suppressing signals, Dr. Delgoffe emphasized. This new oncolytic virus approach is a way to “turn the tide,” he said, and “convert a resistant tumor into a sensitive one.”
Overcoming oncolytic virus limitations
A major rate-limiting factor with oncolytic virus continues to be the need to inject them directly into tumors, Dr. Gulley said. Injection is relatively easy for cancers in the skin, such as melanoma, he continued, but it's much more difficult for tumors located deeper in the body.
So to expand their use, researchers need to find ways to make oncolytic viruses that are “logistically easier to give to patients,” Dr. Gulley said.
Dr. Delgoffe and his team have licensed their modified oncolytic virus to a biotechnology company for further development into a clinical agent. He said he hopes that alterations to the treatment can be made that allow it to be delivered by infusion into the blood stream so it can get to hard-to-reach tumors.