Frederick National Laboratory for Cancer Research: A Unique Resource for the Biomedical Research Community
, by Douglas R. Lowy, M.D.
The Frederick National Laboratory for Cancer Research (FNLCR) is a state-of-the-art research facility located about an hour’s drive from Washington, DC. Its origins date back to the 1971, when President Nixon announced the conversion of part of Fort Detrick into a cancer research center. The Frederick Cancer Research Center opened the following year.
Today, FNLCR employs more than 2,400 people, including scientists and technicians who are working on innovations to prevent and treat cancer, HIV/AIDS, and emerging infectious diseases like COVID-19.
In 2012, FNLCR was given its current name. It is the only US National Laboratory dedicated solely to biomedical research. In recognition of the 10 years since this facility has been named a national laboratory, NCI Principal Deputy Director Douglas R. Lowy, M.D., discusses some of the lab’s initiatives to support cancer research and looks ahead to the next 10 years.
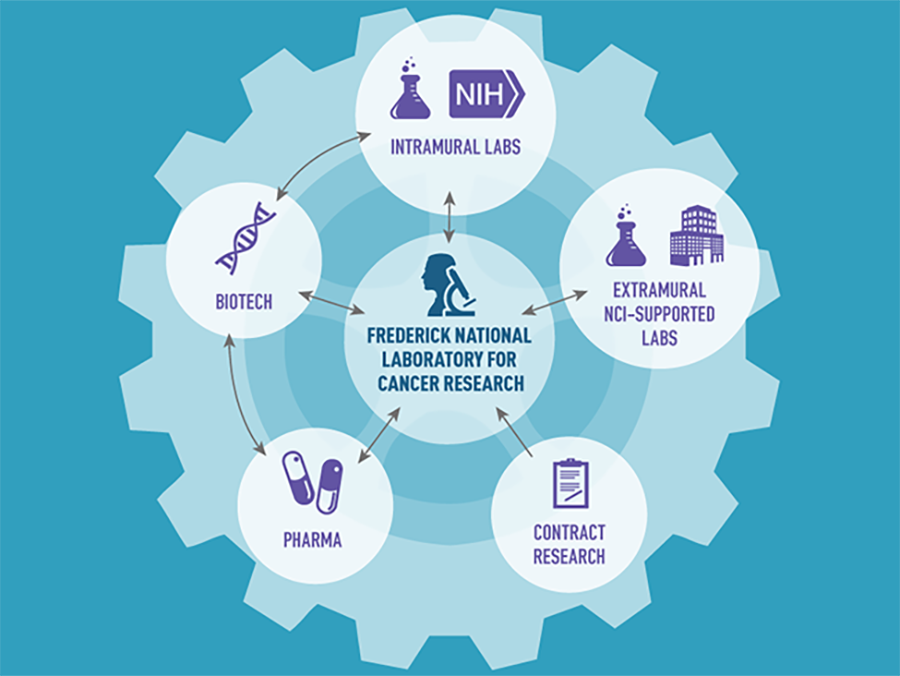
Rep. Mike Simpson (R-ID) often says that the National Institutes of Health (NIH) is Washington’s best-kept secret. But if NIH is Washington's best-kept secret, even fewer people know about the Frederick National Laboratory for Cancer Research (FNLCR)—or at least understand or appreciate its role in advancing our progress against cancer. Yet, FNLCR has contributed to many important research accomplishments.
Over the past decade, this government-owned, contractor-operated research center has launched several high-priority NCI projects that have had direct and immediate impacts on cancer research. They include the RAS Initiative, the Molecular Characterization Lab, and the National Cryo-Electron Microscopy Facility, among many others in its vast research portfolio.
To date, FNLCR has had a hand in the development of 170 unique biopharmaceutical drugs, 150 genetically engineered cancer models, and 150 nanoparticles. Much of this work involves partnerships, including with academic research labs, cancer centers, and pharmaceutical and biotechnology companies.
Even though “cancer research” is in its name, FNLCR’s areas of focus extend well beyond cancer. The lab has, for example, contributed its know-how to tackling some of the most urgent public health challenges of our day, including COVID-19. FNLCR helped with a clinical trial that contributed to the approval of the first treatment for COVID-19.
And in 2021, a monoclonal antibody discovered and developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and manufactured for clinical trials by a NIAID program at FNLCR received FDA approval to treat Ebola virus in adults and children. About two-thirds of FNLCR’s operating costs are funded by NCI, which oversees the lab. The remaining third is funded primarily by NIAID.
In addition to supporting NCI and NIAID, FNLCR also supports other NIH institutes and serves as a shared resource for the biomedical research community. The facility maintains more than 15 million biological specimens, frozen tumor samples, research reagents, and genetically engineered mouse models of human cancers, which are available for use by thousands of researchers around the country either free of charge or for a very small fee.
In recognition of FNLCR’s 10th anniversary, it’s an appropriate time to crack open this unheralded vault in Frederick, Maryland, and offer a peek into the lab’s unique and essential role in making progress against cancer and other public health challenges. And if past is indeed prologue, there are many more research accomplishments to come.
Reinvigorating efforts against RAS
Researchers have known for decades that a family of genes known as RAS—KRAS in particular—are directly involved in fueling the development and growth of a large percentage of hard-to-treat cancers. But nobody could find a way to get a drug to lock onto mutant KRAS proteins and block their cancer-promoting behaviors. KRAS appeared to be, as many frustrated researchers called it, “undruggable.”
So in 2013, NCI launched the RAS initiative, an all-out effort to solve the RAS puzzle—to develop and organize the research needed to find ways to block the activity of RAS proteins. With its advanced technologies and research infrastructure, FNLCR was the ideal homebase for this major effort.
Specifically, FNLCR is at the center of a “hub-and-spoke partnership model” to unite scientists conducting RAS-related research. The facility serves as the hub for different research groups across the country that are supported in part by the larger RAS Initiative. FNLCR also supports the work of RAS researchers at NCI, extramural institutions funded by NCI, and biotech and pharmaceutical companies. To help increase the efficiency of RAS research, FNLCR has also developed a suite of reagents—chemicals and other materials specifically developed to accelerate RAS-related research—that is available to all investigators studying RAS.
FNLCR has also served as a physical hub for bringing the community of RAS researchers together. For example, it has hosted several in-person and virtual RAS symposia, with some drawing more than 1,700 people.
I think it’s fair to say that the decision to launch this initiative and house it at FNLCR has paid off. It has invigorated RAS research and fueled a spirit of collaboration, bringing together academic researchers and pharmaceutical companies in a successful effort to develop RAS-targeted drugs. In just the past 2 years, in fact, FDA has approved two drugs that target an altered form of KRAS. Numerous other KRAS-targeted therapies have been developed and are showing promise.
Supporting NCI-MATCH and cutting-edge technologies
The Molecular Characterization Laboratory at FNLCR does a large volume of genomic sequencing, and, as a result, it has played an important role in NCI-MATCH (NCI-Molecular Analysis for Therapy Choice), a precision medicine cancer treatment clinical trial.
NCI-MATCH was paradigm shifting in several ways. It has been the largest trial to enroll patients based on the molecular abnormalities in patients’ tumors rather than on their specific type of cancer. Because of this unique design, the number of people eligible for the trial was enormous, and no single facility would have had the capacity to sequence all the tumors.
FNLCR coordinated the sequencing efforts to ensure high agreement on genomic testing results between the different sequencing centers. So, whether you sequenced a tumor in Texas or in St. Louis, you could expect to get the same result.
Additional NCI precision medicine trials are now being planned, including Combo-MATCH, MyeloMATCH, and ImmunoMatch, all of which will also rely on the Molecular Characterization Laboratory’s genomic sequencing capabilities to match people with treatments based on genetic changes in their cancer.
The Molecular Characterization Laboratory also performs the genetic sequencing of tumor models that are included in the NCI Patient-Derived Models Repository (PDMR), which is maintained by FNLCR.
These models of different cancer types were developed from tumor tissue samples provided by people participating in clinical trials. The collection, which also includes models of some rare cancers, can be accessed by anyone in the scientific community for use in their drug discovery and development studies. Although the PDMR is still under development, such a repository would be unlikely to be established without the infrastructure and resources of a facility such as FNLCR.
FNLCR also supports cancer research through the National Cryo-Electron Microscopy Facility, which houses advanced microscopy instruments that cancer researchers from across the country can use to precisely map the structure of cancer-related proteins, viruses, and other molecules at a resolution that had been previously unattainable.
As just one example, recently, multiple research groups have used the cryo-EM facility to determine the precise structure of a key protein linked to neurofibromatosis, a condition that leads to the development of noncancerous tumors in areas like the brain and spinal cord.
With such detailed structures in hand, scientists now have a roadmap for designing drugs that can disrupt the behavior of this and other rogue proteins that drive tumor development.
Since NCI launched the National Cryo-EM Facility in May 2017, approximately 100 investigators from 50 institutions have used the facility for their research studies.
Pivoting to fight COVID-19
FNLCR’s cutting-edge technologies and expertise also enable the federal government to quickly respond to emerging public health challenges, such as COVID-19.
During the pandemic, FNLCR worked closely with NIAID, the Centers for Disease Control and Prevention (CDC), and several academic medical centers on a clinical trial that contributed to FDA’s decision to approve the antiviral drug remdesivir (Veklury) for the treatment of severe COVID-19. This international trial took less than a month to launch at the beginning of the pandemic.
Being able to stand up a trial of that magnitude and complexity in such a short time period is a remarkable accomplishment.
In addition, when the COVID-19 pandemic started, some of the resources of FNLCR’s HPV Serology Lab, which develops tests to detect the presence of antibodies to the human papillomavirus (HPV) for HPV vaccine trials, were used to help evaluate tests to detect the presence of antibodies to SARS-CoV-2, the virus that causes COVID-19.
FDA asked FNLCR to evaluate the COVID-19 test kits that were being submitted to FDA for emergency use authorization. The lab ended up evaluating more than 100 different commercial tests, and FDA used these results to decide whether to grant these test kits emergency use authorization. FNLCR experts were also asked to develop a national serology standard and validation panels for SARS-CoV-2 antibody tests, which we did in collaboration with the CDC and NIAID.
Looking ahead to the next 10 years
Over the next 10 years, NCI will launch other signature projects at FNLCR. Given its status as the only national laboratory dedicated to biomedical research, FNLCR may also be a resource to be tapped as part of initiatives launched under the Advanced Research Projects Agency for Health (ARPA-H).
We know a lot more about cancer than we did 10 years ago, but there's still so much we don't understand. We need fundamental research to make breakthroughs that will improve cancer prevention and treatment, and I’m confident that over the next 10 years, and more, FNLCR will contribute to some of those advances and continue to be an important part of our progress.