Pembrolizumab Improves Survival in Advanced Triple-Negative Breast Cancer
, by Edward Winstead
Adding the immunotherapy drug pembrolizumab (Keytruda) to chemotherapy can help some patients with advanced triple-negative breast cancer live longer than if they received chemotherapy alone, new results from a clinical trial show.
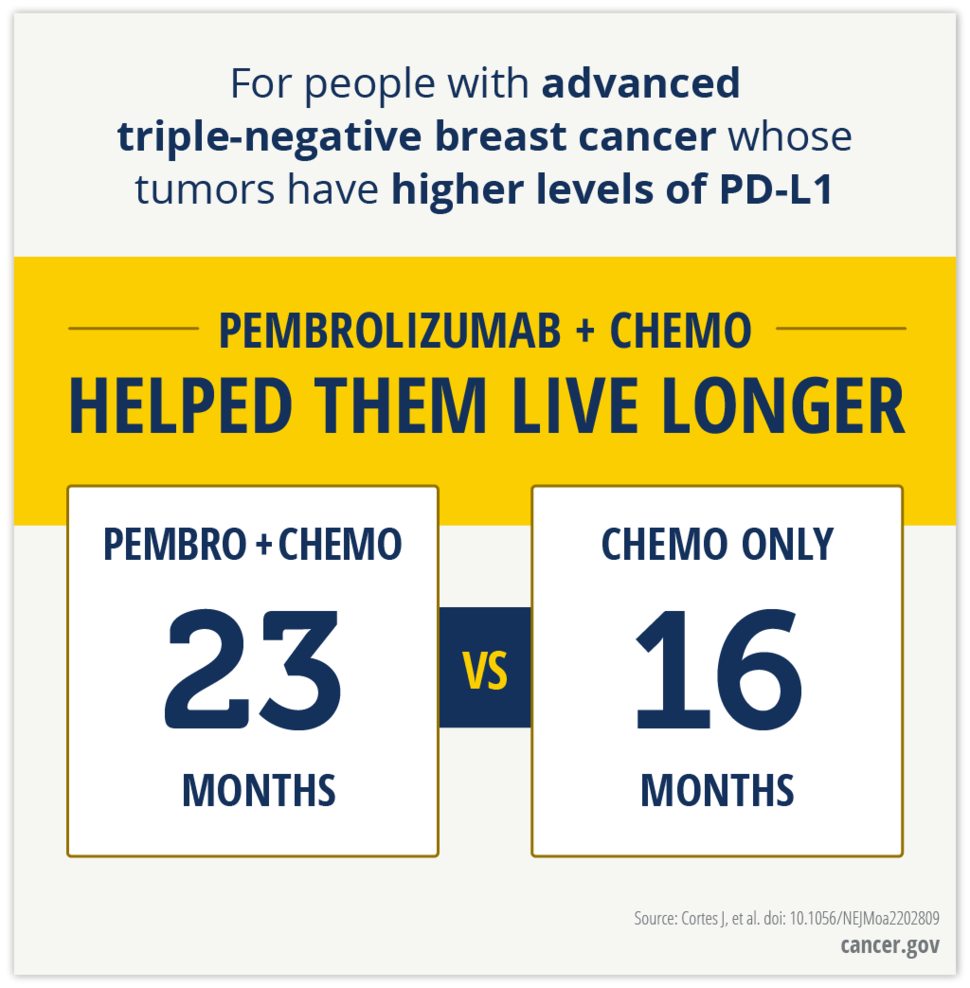
In the trial, KEYNOTE-355, overall survival improved only among patients whose tumors had relatively high levels of the PD-L1 protein—a PD-L1 combined positive score of at least 10.
Among patients with this combined positive score, median overall survival was 23.0 months for those who received pembrolizumab and chemotherapy versus 16.1 months for those who received chemotherapy alone. These results are based on a median follow-up of 44 months.
Javier Cortés, M.D., Ph.D., of the International Breast Cancer Center, Barcelona, Spain, and his colleagues reported their findings July 21 in the New England Journal of Medicine.
In an earlier analysis of interim data from the trial, Dr. Cortés and his colleagues had reported that among patients with a PD-L1 combined positive score of at least 10, the combination of pembrolizumab and chemotherapy improved progression-free survival compared with chemotherapy alone.
Based on those results, in 2020 the Food and Drug Administration (FDA) approved the combination therapy for patients with advanced triple-negative breast cancer whose tumors have a PD-L1 combined score of at least 10.
The approval covers patients with cancer that cannot be removed surgically (unresectable), that has spread to tissue near the breast but not to other parts of the body (locally advanced), or that has spread to other parts of the body (metastatic).
The new KEYNOTE-355 results confirm that pembrolizumab is a “breakthrough” treatment for triple-negative breast cancer, wrote Xavier Pivot, M.D., Ph.D., of the Institute of Cancerology Strasbourg, Strasbourg, France, in an accompanying editorial.
New treatments needed for advanced triple-negative breast cancer
Triple-negative breast cancer tends to be more aggressive, harder to treat, and more likely to recur than other forms of the disease, such as hormone receptor–positive or HER2-positive breast cancers.
Conventional chemotherapy drugs have not been effective against triple-negative breast cancer, and new treatment options are needed, said Jung-Min Lee, M.D., of the Women’s Malignancies Branch in NCI’s Center for Cancer Research.
In the KEYNOTE-355 trial, 847 patients with advanced (unresectable, locally advanced, or metastatic) triple-negative breast cancer were randomly assigned to receive chemotherapy plus placebo or chemotherapy plus pembrolizumab.
The study assessed the amount of time before the disease worsened (progression-free survival) and overall survival in all patients, in those with PD-L1 combined positive scores of 1 or more, and in those with combined positive scores of 10 or more. The trial was funded by Merck, the manufacturer of pembrolizumab.
The PD-L1 combined positive score is essentially a measure of the extent to which cells in a tumor produce PD-L1, the immune checkpoint protein that pembrolizumab targets. By blocking immune checkpoints, pembrolizumab and other immune checkpoint inhibitors unleash the immune system against cancer cells.
Among patients with a combined PD-L1 score of more than 1, pembrolizumab did not improve median overall survival, which was 17.6 months in the pembrolizumab–chemotherapy group and 16.0 months in the chemotherapy-alone group.
Among patients with a combined PD-L1 score of 10 or more, at 18 months after starting treatment, about 58% of patients in the combination-treatment group were still alive, compared with about 45% of those in the chemotherapy-alone group.
The incidence of treatment-related side effects, including serious side effects, was similar between the two groups of patients in the study.
The most common side effects in both groups were a drop in red blood cells, a lower-than-normal number of white blood cells, and nausea. These side effects are typically associated with chemotherapy, and adding pembrolizumab to chemotherapy did not increase the incidence of side effects among patients, the study authors wrote.
Research progress and future questions
After the 2020 approval of the combination of pembrolizumab and chemotherapy for advanced triple-negative breast cancer, FDA approved the combination therapy for people with early-stage disease in 2021.
That approval was based on results from a different trial, KEYNOTE-522. In that study, patients with high-risk, early-stage triple-negative breast cancer benefited from pembrolizumab given with chemotherapy before surgery, and then continued as a single agent as an additional, or adjuvant, treatment after surgery.
“This is an exciting time” for research on triple-negative breast cancer, said Dr. Lee. “We have now seen a benefit from an immune checkpoint inhibitor and chemotherapy” in a subgroup of patients in both the advanced and early stages of the disease.
Dr. Lee cautioned, however, that more than half of all patients with triple-negative breast cancer have PD-L1 combined positive scores of less than 10, so more work is needed to find effective treatments for these patients.
In his editorial, Dr. Pivot noted that people diagnosed with triple-negative breast cancer are not a homogeneous group. Future studies, he added, will try to identify which individuals are more or less likely to benefit from pembrolizumab.