NCI Part of Federal Effort to Evaluate Antibody Tests for Novel Coronavirus
, by NCI Staff
As part of a collaboration with the Food and Drug Administration (FDA) and several other government agencies and academic medical centers, NCI is evaluating commercially available antibody tests for SARS-CoV-2, the novel coronavirus that causes COVID-19.
NCI has already assessed several of the tests and has provided the findings to FDA.
While “cancer research and cancer care remain job number one at NCI,” said NCI Director Norman Sharpless, M.D., “NCI has unique research capabilities and capacities. So, to help in this public health crisis, we believe, is a moral obligation.”
Because of its robust research infrastructure, including expertise in human papillomavirus (HPV) vaccines and an advanced serology laboratory, part of NCI’s Frederick National Laboratory for Cancer Research (FNLCR) has shifted its focus to provide independent testing and validation of SARS-CoV-2 antibody tests.
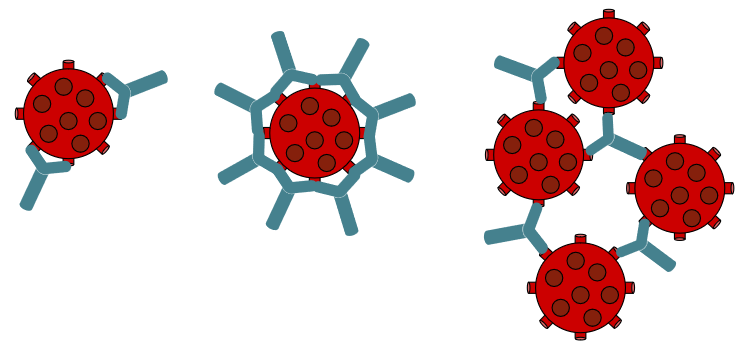
Antibody tests, also called serology tests, can be used to identify whether individuals have antibodies against SARS-CoV-2 in their blood. Antibodies are proteins made by the immune system in response to an infection. If someone has antibodies to the virus, it means that the person is, or was, infected.
At the time of publication, FDA has authorized emergency use of twelve different antibody tests, but many more tests are available commercially that haven’t been reviewed by the agency. On May 4, the agency updated its earlier policy guidance on antibody tests for commercial test manufacturers related to emergency use authorizations and providing specific clinical performance expectations for these tests.
“With support from Congress, NCI is working with the FDA and other government agencies to rapidly and rigorously characterize the performance of serology assays,” said Douglas Lowy, M.D., NCI’s principal deputy director and a leading expert on HPV. The results of serology tests, along with other relevant information, such as a person’s clinical history or other diagnostic test results, “can help determine who in the community has had a prior COVID-19 infection,” Dr. Lowy said.
“Determining the next steps in our response to COVID-19 is partially dependent on an accurate assessment of our national efforts thus far, and the quality of data for making this decision is dependent on accurate testing products,” explained FDA Commissioner Stephen Hahn, M.D., in a statement.
Validated serology tests are also crucial for further studies of COVID-19, including studies of disease prevalence (called seroprevalence), immunity, and candidate vaccines, explained Ligia Pinto, Ph.D., who is leading the antibody testing work. Dr. Pinto directs FNLCR’s Vaccine, Immunity, and Cancer Program and the HPV Serology Laboratory, which develops, optimizes, and validates serology tests for HPV.
A Group Effort to Evaluate SARS-CoV-2 Antibody Tests
Reviewing the accuracy of SARS-CoV-2 antibody tests is a collaborative effort between FDA, NCI, the Centers for Disease Control and Prevention (CDC), the National Institute of Allergy and Infectious Diseases (NIAID), the Biomedical Advanced Research and Development Authority in the Department of Health and Human Services, Mount Sinai Health System, and others.
NCI has an inherent interest in contributing to the COVID-19 response because people with cancer are more likely to get very sick and die from the disease, Dr. Lowy said.
In addition, FNLCR has unique resources and capabilities that are tailor-made for public health emergencies like the current pandemic, Dr. Sharpless explained.
And its scientists are no strangers to working on public health emergencies. Alongside experts from NIAID, they have helped respond to earlier virus epidemics, including SARS, Ebola, and Zika.
“A lot of the same principles apply when you go from one type of serology assay to another,” Dr. Pinto explained. “The work we have done with HPV serology has really prepared us to evaluate and validate technologies for the COVID-19 pandemic.”
Are SARS-CoV-2 Antibody Tests Accurate?
The main goal of this effort is to determine whether available antibody tests are accurate. Meaning, does a given test pick up SARS-CoV-2 antibodies when they are present in someone’s blood, and not give a signal when they aren’t?
To answer that question, Dr. Pinto’s team runs each test on a set of 110 blood samples, called a “validation panel.” Thirty samples are from people who had a confirmed SARS-CoV-2 infection. Another 80 samples were taken from people before the COVID-19 pandemic started and, therefore, wouldn’t have been infected with SARS-CoV-2. Samples from the panel can be used to test for two types of antibodies: IgG and IgM.
To ensure accuracy, every sample in the validation panel was tested by at least two separate labs. Although the panel may not be representative of all sample types that may be encountered in a large population, it will help inform next steps in the pandemic response, Dr. Pinto said.
Applying each antibody test to a validation panel reveals how often each test gets the right result—what’s known as sensitivity and specificity. False-negative results could lead people to believe that they haven’t been infected when they actually have, potentially preventing them from returning to regular activities, such as work or school.
And false-positive results would cause people to think they have been infected and have developed an immune response, when they haven’t. Incorrect results could also provide a skewed picture of how many people have actually been infected and what the true death rate of COVID-19 is, experts have stressed.
Results from NCI’s validation studies are being provided to FDA on a rolling basis; the agency will use the information to decide how the tests should be used and to inform additional regulatory decisions.
Another goal of this effort is to develop standards for SARS-CoV-2 serology tests, Dr. Pinto added. If everyone working with serology tests uses the same standards, “then results can be compared more easily between different studies and even different assays,” she explained.
Her team has experience there too: They are leading an initiative to standardize HPV serology tests used to monitor immune responses to HPV vaccines in patients participating in clinical trials.
What Does Having SARS-CoV-2 Antibodies Mean?
“At the moment, a positive [antibody] test only means that person currently has or has had a SARS-CoV-2 infection,” said Dr. Lowy.
“We don’t yet know whether someone who has recovered from the infection and is now antibody positive is resistant to reinfection. The answer to that important question, which we hope will be positive, is the subject of ongoing research,” he added.
Unlike the “swab tests” used to diagnose COVID-19, which look for pieces of the virus itself, antibody tests can’t be used to diagnose the disease or determine if someone is infectious.
In the future, antibody tests may be able to provide important information about the immune response to COVID-19. For instance, how quickly does the body make antibodies against SARS-CoV-2? What is the relationship between that immune response and COVID-19 progression? What parts of the virus do antibodies bind to?
“You need accurate and highly reproducible antibody tests to be able to answer these questions,” Dr. Pinto said. “We are very interested in using robust and well-validated assays to do clinical research to understand the immune response to this virus in healthy individuals, as well as in cancer patients and those with other underlying conditions,” she added.
And, as is the case for HPV, validated SARS-CoV-2 antibody tests are also needed for studies of potential COVID-19 vaccines. The tests are used to see if people who got the vaccine in clinical trials have protective antibodies in their blood and how long those antibodies last.
Public Health Uses for Antibody Tests
Beyond translational and clinical research, antibody tests have several potential public health uses.
A critical role for the tests is to help determine the true scope of the pandemic. NIAID, NCI, and other NIH institutes have begun a study of 10,000 volunteers using an antibody test to quantify previously undetected cases of COVID-19. The study will determine the percentage of people who have been infected without knowing it because they had mild or no symptoms or didn’t get diagnostic testing when they were sick.
In addition, CDC is helping states and localities determine COVID-19 prevalence based on antibody testing.
The tests can also help find antibodies that may potentially be used as blood-based therapies. FDA is leading a national effort to develop antibody-based therapies from the blood of volunteers who have recovered from COVID-19. There is some evidence that two kinds of antibody-rich blood products, called convalescent plasma and hyperimmune globulin, can benefit people who are sick with COVID-19.
If studies suggest that SARS-CoV-2 antibodies are a sign of lasting immunity, public health experts believe antibody tests could also help determine who can safely return to work or school, and would be especially helpful for frontline workers who are more likely to be exposed to infected individuals.