Cancer Genomics Overview
The field of cancer genomics is constantly growing and changing, fueled by the development of new laboratory and computational technologies for interrogating the molecular and cellular details of cancer.
By characterizing not only the DNA, but also gene expression, proteins, and many other molecular features of cancer, researchers can identify key changes that may cause the disease. By taking advantage of new types of data, improvements in data quality, and reductions in cost for sequencing, researchers are gaining a fuller understanding of cancer.
Once cancer-causing changes are identified, scientists can gain a better understanding of the molecular basis of cancer growth, metastasis, and drug resistance. This is done using clinical data that describes how patients responded to cancer treatment, laboratory experiments using cell lines and model organisms, and big data analysis techniques. Putting large genomic datasets together and sharing them with researchers worldwide is an increasingly important strategy for cancer research, as this boosts the power of the data and opens new opportunities for discovery. Scientists at the National Institutes of Health (NIH), as well as around the world, are working diligently to identify genetic changes underlying cancer, determine their roles in tumor development and metastasis, and harness these findings to fight cancer.

Opportunities in Research - Building on Discoveries in Cancer Genomics
Louis Staudt, M.D., Ph.D., director of the Center for Cancer Genomics, discusses the future of cancer research and building on discoveries in cancer genomics.
Importance of Cancer Genomics in Precision Cancer Medicine
Genomic information about cancer is leading to better diagnoses and treatment strategies that are tailored to patients’ tumors, an approach called precision medicine. As a result of research into the genomic changes associated with cancer, drugs have been developed to fight the disease in several ways:
- Inhibiting enzymes that trigger the abnormal growth and survival of cancer cells
- Blocking aberrant gene expression characteristic of cancer cells
- Halting molecular signaling pathways that are in overdrive in cancer cells
These “targeted therapies” specifically combat characteristics of cancer cells that are different from normal cells of the body. This makes them less likely to be toxic for patients compared to other treatments such as chemotherapy and radiation that can kill normal cells. There are several examples of precision medicine already in clinical use:
- Imatinib (Gleevec) inhibits overactivity of a protein (called Bcr-Abl tyrosine kinase) in patients whose leukemia is caused by a particular chromosomal rearrangement.
- Trastuzumab (Herceptin) controls a hyperactive signaling pathway (HER2 tyrosine kinase) caused by multiple copies of the HER2 gene in a subtype of breast cancers.
- Erlotinib (Tarceva) and gefitinib (Iressa) both restrict activation of a protein (epidermal growth factor, or EGFR), which is abnormally active in a subset of lung cancers due to mutations in the protein.
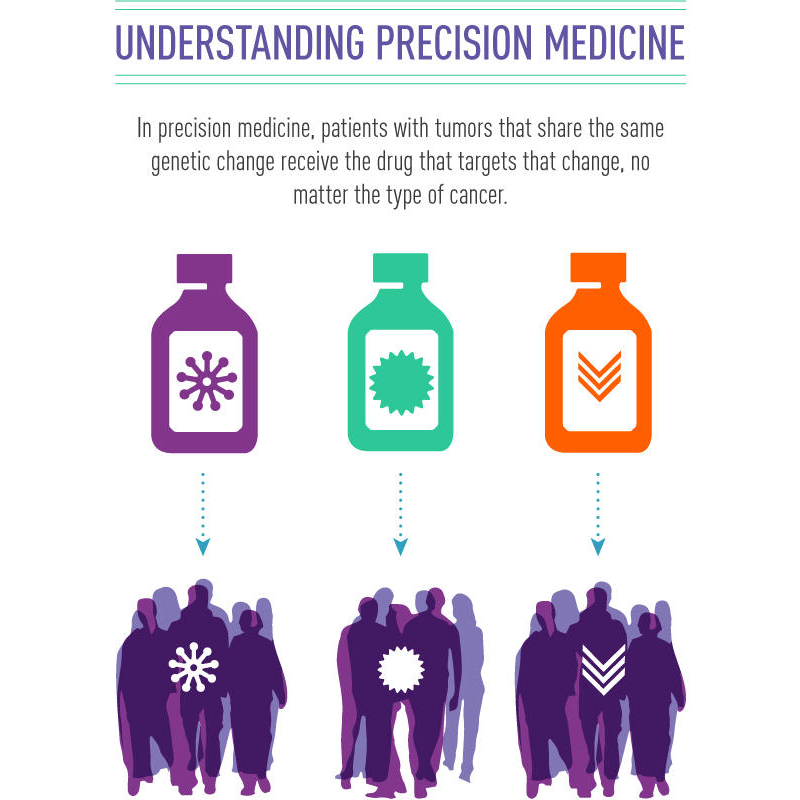
Using the genetic changes in a patient’s tumor to determine their treatment is known as precision medicine.
Credit: National Cancer Institute
Cancer genomics research also contributes to precision medicine by defining cancer types and subtypes based on their genetics. This molecular taxonomy of cancer can provide patients with a more precise diagnosis, and therefore a more personalized treatment strategy. There are several ways in which the molecular definition of cancer already benefits patients:
- Breast cancer is classified based on molecular characteristics into distinct subgroups – Luminal A, Luminal B, Triple-negative/basal-like, and HER2 type – that vary in their aggressiveness and respond differently to therapies. Breast cancer patients may benefit from a diagnosis and treatment guided by knowledge of their tumor's molecular subtype.
- Diffuse large B cell lymphoma can be subdivided into the ABC and GCB subtypes by genomic profiling, identifying patients that respond differently to current chemotherapy regimens and to molecularly targeted therapies.
- In 2013, The Cancer Genome Atlas project identified four subtypes of endometrial cancer – POLE ultramutated, microsatellite instability (MSI) hypermutated, copy-number (CN) low, and CN high – that correlate with patient survival. This research has already given rise to new clinical trials that investigate how these subtypes can improve the future of endometrial cancer care.
- Lung cancer patients who have a gene fusion involving the ROS1 gene often respond well to treatment with a targeted therapy called crizotinib. In these cases, the disease is best defined and treated based on its unique genetic change.