How does T-cell transfer therapy work against cancer?
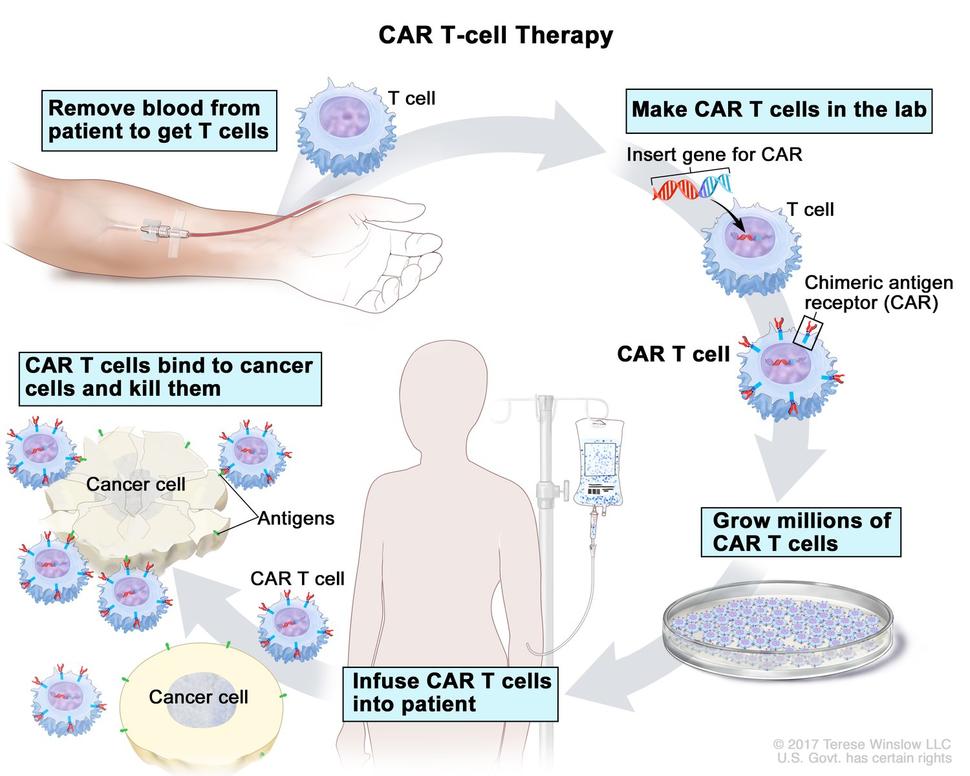
T-cell transfer therapy is a type of immunotherapy that makes your own immune cells better able to attack cancer. There are two main types of T-cell transfer therapy: tumor-infiltrating lymphocytes (or TIL) therapy and CAR T-cell therapy. Both involve collecting your own immune cells, growing large numbers of these cells in the lab, and then giving the cells back to you through a needle in your vein. T-cell transfer therapy is also called adoptive cell therapy, adoptive immunotherapy, and immune cell therapy.
The process of growing your T cells in the lab can take 2 to 8 weeks. During this time, you may have treatment with chemotherapy and, maybe, radiation therapy to get rid of other immune cells. Reducing your immune cells helps the transferred T cells to be more effective. After these treatments, the T cells that were grown in the lab will be given back to you via a needle in your vein.
- TIL therapy uses T cells called tumor-infiltrating lymphocytes that are found in your tumor. Doctors test these lymphocytes in the lab to find out which ones best recognize your tumor cells. Then, these selected lymphocytes are treated with substances that make them grow to large numbers quickly.
- The idea behind this approach is that the lymphocytes that are in or near the tumor have already shown the ability to recognize your tumor cells. But there may not be enough of them to kill the tumor or to overcome the signals that the tumor is releasing to suppress the immune system. Giving you large numbers of the lymphocytes that react best with the tumor can help to overcome these barriers.
- CAR T-cell therapy is similar to TIL therapy, but your T cells are changed in the lab so that they make a type of protein known as CAR before they are grown and given back to you. CAR stands for chimeric antigen receptor. CARs are designed to allow the T cells to attach to specific proteins on the surface of the cancer cells, improving their ability to attack the cancer cells.
What cancers are treated with T-cell transfer therapy?
A TIL therapy called lifileucel (Amtagvi) has been approved by the Food and Drug Administration (FDA) to treat melanoma. And it has produced promising findings in other cancers, such as cervical squamous cell carcinoma and cholangiocarcinoma. However, this treatment is still experimental for those cancers.
Six CAR T-cell therapies have been approved by the FDA for blood cancers.
- axicabtagene ciloleucel (Yescarta)
- brexucabtagene autoleucel (Tecartus)
- ciltacabtagene autoleucel (Carvykti)
- idecabtagene vicleucel (Abecma)
- lisocabtagene maraleucel (Breyanzi)
- tisagenlecleucel (Kymriah)
CAR T-cell therapy has also been studied for the treatment of solid tumors, including breast and brain cancers, but use in such cancers is still experimental.
What are the side effects of T-cell transfer therapy?
T-cell transfer therapy can cause side effects, which people experience in different ways. The side effects you may have and how serious they are will depend on how healthy you are before treatment, your type of cancer, how advanced it is, the type of T-cell transfer therapy you are receiving, and the dose.
Doctors and nurses cannot know for sure when or if side effects will occur or how they will affect you. So, it is important to know which signs to look for and what to do if you start to have problems.
CAR T-cell therapy can cause a serious side effect known as cytokine release syndrome. This syndrome is caused when the transferred T cells, or other immune cells responding to the new T cells, release a large amount of cytokines into the blood.
Cytokines are immune substances that have many different functions in the body. A sudden increase in their levels can cause:
- fever
- nausea
- headache
- rash
- rapid heartbeat
- low blood pressure
- trouble breathing
Most people have a mild form of cytokine release syndrome. But in some people, it may be severe or life-threatening.
Also, although CAR T cells are designed to recognize proteins that are found only on cancer cells, they can also sometimes recognize normal cells. Depending on which normal cells are recognized, this can cause a range of side effects, including organ damage.
TIL therapy can cause capillary leak syndrome. This syndrome causes fluid and proteins to leak out of tiny blood vessels and flow into surrounding tissues, resulting in dangerously low blood pressure. Capillary leak syndrome may lead to multiple organ failure and shock.