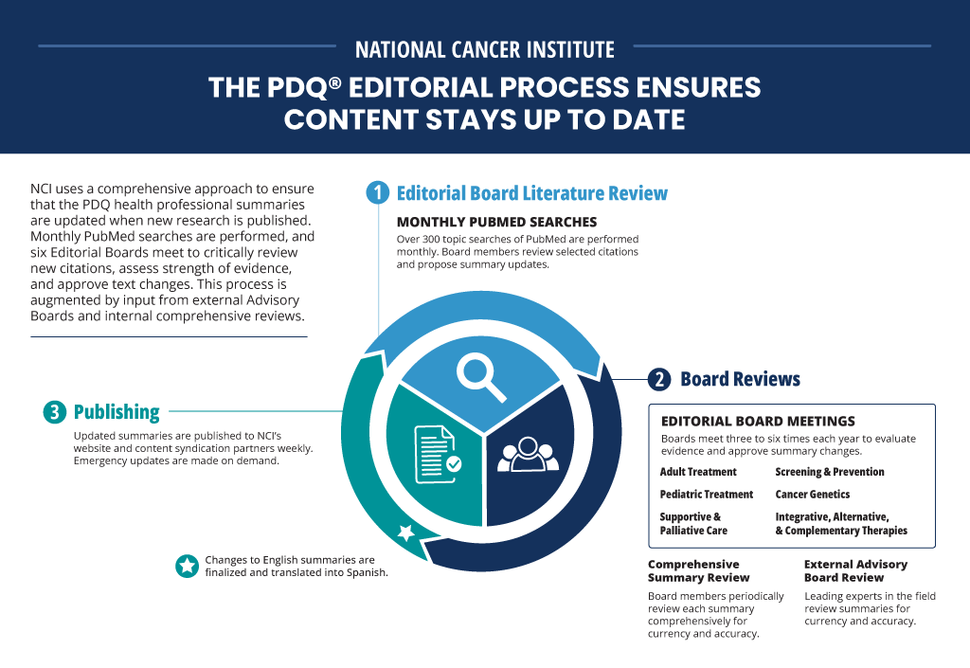
Core Editorial Board Processes
The dynamic editorial processes used for writing and updating the PDQ health professional cancer information summaries ensure that information is continuously updated as new research becomes available.
The main processes include monthly PubMed literature surveillance, strength of evidence assessments, and summary development and maintenance through the PDQ Editorial Boards and Working Groups.
PubMed Searches
Each month, over 300 topic-based searches of the peer-reviewed biomedical literature in NLM’s PubMed database are conducted. These searches yield a pool of citations that is screened through a citation review process to identify studies of the highest level of evidence and the greatest relevance to each PDQ Editorial Board.
Citation Review Process
- The PubMed search results are imported into a system known as the PDQ Editorial Board Management System (EBMS) where medical librarians conduct an initial review and exclude citations from journals published in a foreign language and citations from journals (identified by PDQ Editorial Board members) that generally do not publish strong research studies.
- The remaining citations are associated with one or more PDQ cancer information summary topics and made available in the EBMS for the next step in the citation review process. Abstracts of the corresponding research articles and selected full text articles are reviewed by the Editorial Board Managers. Recommendations are made about which citations meet the evidence standards of the Board and should be forwarded to PDQ Editorial Board members.
- The selected full-text articles are then made available electronically to the appropriate Editorial Board member(s) for their review. The criteria Board members use to select citations for the PDQ Editorial Boards to review are specific for each Board. Learn more about how each Board assesses the evidence.
- As Board members review new citations, they decide whether any changes to the PDQ summary are required, whether the article should be cited in a summary, whether the article replaces an older study citing new information, whether the text needs to be revised, and whether the citation deserves discussion at a future Editorial Board meeting. In determining whether citations should be accepted or rejected for inclusion in a PDQ health professional summary, Board members are asked to consider study design and power, contribution of new primary data, potential study biases and missing outcomes data, and importance to clinical practice.
- The Board members then record their decisions and upload any proposed summary changes to the EBMS.
- The Editorial Board Manager reviews the decisions and proposed changes and uses this information to develop agendas for the Editorial Board meetings.
Editorial Board Review
The six core PDQ Editorial Boards (Adult Treatment, Pediatric Treatment, Screening and Prevention, Supportive and Palliative Care, Cancer Genetics, and Integrative, Alternative, and Complementary Therapies) meet three to six times per year.
Each PDQ summary is assigned to at least one Editorial Board member as lead reviewer. Learn more about the PDQ Editorial Boards.
New citations that result in changes to a PDQ summary are almost always discussed at an Editorial Board meeting. For each article discussed at a Board meeting, the strengths and weaknesses of the study are considered, and the study is assigned a Level of Evidence based on the study design, when appropriate.
The decision to include an article and the text describing it is made by the entire Board.
For some topics with a significant amount of literature, Working Groups have been formed to meet outside of the regular Board meetings to assess the literature and make recommendations to the full Editorial Board about the highest quality evidence.
Comprehensive Summary Reviews
In addition to regular PDQ Editorial Board meeting reviews, the lead reviewers for each cancer information summary comprehensively review the entire summary every 2-3 years for accuracy, currency, and readability.
External Editorial Advisory Boards
Each core PDQ Editorial Board is supported by a corresponding external Editorial Advisory Board. Every PDQ cancer information summary is sent to at least one Advisory Board member every 2-3 years for external review. Advisory Board members suggest changes; however, final decisions on revisions are made by the core Editorial Board.