Peripheral T-Cell Non-Hodgkin Lymphoma Treatment (PDQ®)–Health Professional Version
General Information About Peripheral T-Cell Non-Hodgkin Lymphoma
The non-Hodgkin lymphoma (NHL) T-cell lymphomas are a heterogeneous group of T-cell lymphoproliferative malignancies, which account for less than 15% of NHLs.[1] About 85% of NHL cases are B-cell lymphomas. For more information, see Indolent B-Cell Non-Hodgkin Lymphoma Treatment and Aggressive B-Cell Non-Hodgkin Lymphoma Treatment.
T-cell lymphoma can be divided into cutaneous T-cell lymphoma (CTCL), peripheral T-cell lymphoma (PTCL), and T-cell lymphoblastic lymphoma/acute lymphocytic leukemia (T-LBL/ALL).
T-LBL/ALL arises from very early T cells, often involves the thymus, and is more common in young adults. The lymphoma form is often treated similarly to the leukemia form. For more information, see Acute Lymphoblastic Leukemia Treatment.
CTCL starts in the skin and includes mycosis fungoides, Sézary syndrome, primary cutaneous anaplastic large cell lymphoma, and others. For more information, see Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment.
PTCL originates from mature T cells. It usually arises from lymphoid tissues but can spread to other organs. Subsets of PTCL include anaplastic large cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), extranodal natural killer/T-cell lymphoma (ENK/TCL), PTCL not otherwise specified (PTCL-NOS), enteropathy-associated T-cell lymphoma (EATL), hepatosplenic T-cell lymphoma (HSTCL), adult T-cell leukemia/lymphoma (ATL), T-cell prolymphocytic leukemia (T-PLL), and others.
Incidence and Mortality
T-cell lymphomas make up less than 15% of NHL cases. Most T-cell lymphoma subtypes are associated with worse outcomes than those of B-cell lymphomas.[1]
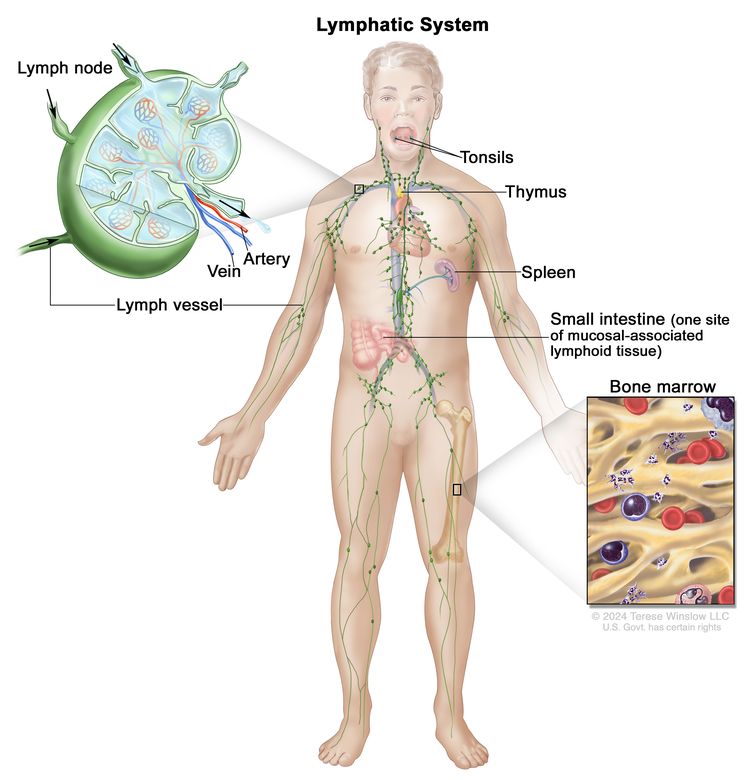
Anatomy
NHL usually originates in lymphoid tissues.
Prognosis and Survival
Prognosis in PTCL varies depending on subtype, stage, and other factors. In general, PTCL is associated with a poor prognosis, with a 5-year survival rate of approximately 30% to 40%.[2,3] While outcomes are better for patients with ALK-positive ALCL, with a median 5-year overall survival (OS) closer to 70% to 80%,[2-4] other subsets are associated with worse survival, such as ALK-negative ALCL, AITL, PTCL-NOS, HSTCL, EATL, and ENK/TCL.[5,6]
Unlike B-cell NHLs, which include both indolent and aggressive forms, most PTCLs are considered aggressive.[7] As with most other aggressive lymphomas, PTCLs are often curable with systemic therapy, though effective treatment options are more limited, particularly in the relapsed or refractory setting.[8,9]
Even though existing treatments cure a significant fraction of patients with lymphoma, numerous clinical trials that explore treatment improvements are in progress. If possible, patients can be included in these studies.
In addition to screening for HIV among patients with aggressive lymphomas, active hepatitis B or hepatitis C can be assessed before treatment with chemotherapy.[10,11] Patients with detectable hepatitis B virus (HBV) benefit from prophylaxis with entecavir.[12,13] Patients with a resolved HBV infection (defined as hepatitis B surface antigen-negative but hepatitis B core antibody-positive) are at risk of reactivation of HBV and require monitoring of HBV DNA. Prophylactic nucleoside therapy lowered HBV reactivation from 10.8% to 2.1% in a retrospective study of 326 patients.[14] Prophylaxis for herpes zoster with acyclovir or valacyclovir and prophylaxis for pneumocystis with trimethoprim/sulfamethoxazole or dapsone are usually given to patients receiving combination chemotherapy.
References
- American Cancer Society: Types of T-cell lymphoma. American Cancer Society, 2018. Available online. Last accessed May 13, 2025.
- Vose J, Armitage J, Weisenburger D, et al.: International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 26 (25): 4124-30, 2008. [PUBMED Abstract]
- Ellin F, Landström J, Jerkeman M, et al.: Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 124 (10): 1570-7, 2014. [PUBMED Abstract]
- Sibon D, Fournier M, Brière J, et al.: Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol 30 (32): 3939-46, 2012. [PUBMED Abstract]
- Petrich AM, Helenowski IB, Bryan LJ, et al.: Factors predicting survival in peripheral T-cell lymphoma in the USA: a population-based analysis of 8802 patients in the modern era. Br J Haematol 168 (5): 708-18, 2015. [PUBMED Abstract]
- Foss FM, Horwitz SM, Civallero M, et al.: Incidence and outcomes of rare T cell lymphomas from the T Cell Project: hepatosplenic, enteropathy associated and peripheral gamma delta T cell lymphomas. Am J Hematol 95 (2): 151-155, 2020. [PUBMED Abstract]
- Armitage JO: The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol 92 (7): 706-715, 2017. [PUBMED Abstract]
- Bellei M, Foss FM, Shustov AR, et al.: The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica 103 (7): 1191-1197, 2018. [PUBMED Abstract]
- Lansigan F, Horwitz SM, Pinter-Brown LC, et al.: Outcomes for Relapsed and Refractory Peripheral T-Cell Lymphoma Patients after Front-Line Therapy from the COMPLETE Registry. Acta Haematol 143 (1): 40-50, 2020. [PUBMED Abstract]
- Niitsu N, Hagiwara Y, Tanae K, et al.: Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol 28 (34): 5097-100, 2010. [PUBMED Abstract]
- Dong HJ, Ni LN, Sheng GF, et al.: Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 57 (3): 209-14, 2013. [PUBMED Abstract]
- Huang YH, Hsiao LT, Hong YC, et al.: Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 31 (22): 2765-72, 2013. [PUBMED Abstract]
- Li H, Zhang HM, Chen LF, et al.: Prophylactic lamivudine to improve the outcome of HBsAg-positive lymphoma patients during chemotherapy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 39 (1): 80-92, 2015. [PUBMED Abstract]
- Kusumoto S, Arcaini L, Hong X, et al.: Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 133 (2): 137-146, 2019. [PUBMED Abstract]
Late Effects of Treatment of Peripheral T-Cell Non-Hodgkin Lymphoma
Late effects of treatment of non-Hodgkin lymphoma (NHL) have been observed. Impaired fertility may occur after exposure to alkylating agents.[1] For as many as three decades after diagnosis, patients are at a significantly elevated risk of developing second primary cancers, especially the following:[2-5]
- Lung cancer.
- Brain cancer.
- Kidney cancer.
- Bladder cancer.
- Melanoma.
- Hodgkin lymphoma.
- Acute nonlymphocytic leukemia.
Left ventricular dysfunction was a significant late effect in long-term survivors of high-grade NHL who received more than 200 mg/m² of doxorubicin.[1,6]
Myelodysplastic syndrome and acute myelogenous leukemia are late complications of myeloablative therapy with autologous bone marrow or peripheral blood stem cell support, as well as conventional chemotherapy-containing alkylating agents.[3,7-14] Most of these patients show clonal hematopoiesis even before the transplant, suggesting that the hematologic injury usually occurs during induction or reinduction chemotherapy.[9,15,16] A series of 605 patients who received autologous bone marrow transplant (BMT) with cyclophosphamide and total-body radiation therapy (as conditioning) were followed for a median of 10 years. The incidence of a second malignancy was 21%, and 10% of those malignancies were solid tumors.[17]
A study of young women who received autologous BMT reported successful pregnancies with children born free of congenital abnormalities.[18] Late-occurring venous thromboembolism can occur after allogeneic or autologous BMT.[19]
Some patients have osteopenia or osteoporosis at the start of therapy; bone density may worsen after therapy for lymphoma.[20]
References
- Haddy TB, Adde MA, McCalla J, et al.: Late effects in long-term survivors of high-grade non-Hodgkin's lymphomas. J Clin Oncol 16 (6): 2070-9, 1998. [PUBMED Abstract]
- Travis LB, Curtis RE, Glimelius B, et al.: Second cancers among long-term survivors of non-Hodgkin's lymphoma. J Natl Cancer Inst 85 (23): 1932-7, 1993. [PUBMED Abstract]
- Mudie NY, Swerdlow AJ, Higgins CD, et al.: Risk of second malignancy after non-Hodgkin's lymphoma: a British Cohort Study. J Clin Oncol 24 (10): 1568-74, 2006. [PUBMED Abstract]
- Hemminki K, Lenner P, Sundquist J, et al.: Risk of subsequent solid tumors after non-Hodgkin's lymphoma: effect of diagnostic age and time since diagnosis. J Clin Oncol 26 (11): 1850-7, 2008. [PUBMED Abstract]
- Major A, Smith DE, Ghosh D, et al.: Risk and subtypes of secondary primary malignancies in diffuse large B-cell lymphoma survivors change over time based on stage at diagnosis. Cancer 126 (1): 189-201, 2020. [PUBMED Abstract]
- Moser EC, Noordijk EM, van Leeuwen FE, et al.: Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 107 (7): 2912-9, 2006. [PUBMED Abstract]
- Darrington DL, Vose JM, Anderson JR, et al.: Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol 12 (12): 2527-34, 1994. [PUBMED Abstract]
- Stone RM, Neuberg D, Soiffer R, et al.: Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 12 (12): 2535-42, 1994. [PUBMED Abstract]
- Armitage JO, Carbone PP, Connors JM, et al.: Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol 21 (5): 897-906, 2003. [PUBMED Abstract]
- André M, Mounier N, Leleu X, et al.: Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood 103 (4): 1222-8, 2004. [PUBMED Abstract]
- Oddou S, Vey N, Viens P, et al.: Second neoplasms following high-dose chemotherapy and autologous stem cell transplantation for malignant lymphomas: a report of six cases in a cohort of 171 patients from a single institution. Leuk Lymphoma 31 (1-2): 187-94, 1998. [PUBMED Abstract]
- Lenz G, Dreyling M, Schiegnitz E, et al.: Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J Clin Oncol 22 (24): 4926-33, 2004. [PUBMED Abstract]
- McLaughlin P, Estey E, Glassman A, et al.: Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood 105 (12): 4573-5, 2005. [PUBMED Abstract]
- Morton LM, Curtis RE, Linet MS, et al.: Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 28 (33): 4935-44, 2010. [PUBMED Abstract]
- Mach-Pascual S, Legare RD, Lu D, et al.: Predictive value of clonality assays in patients with non-Hodgkin's lymphoma undergoing autologous bone marrow transplant: a single institution study. Blood 91 (12): 4496-503, 1998. [PUBMED Abstract]
- Lillington DM, Micallef IN, Carpenter E, et al.: Detection of chromosome abnormalities pre-high-dose treatment in patients developing therapy-related myelodysplasia and secondary acute myelogenous leukemia after treatment for non-Hodgkin's lymphoma. J Clin Oncol 19 (9): 2472-81, 2001. [PUBMED Abstract]
- Brown JR, Yeckes H, Friedberg JW, et al.: Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 23 (10): 2208-14, 2005. [PUBMED Abstract]
- Jackson GH, Wood A, Taylor PR, et al.: Early high dose chemotherapy intensification with autologous bone marrow transplantation in lymphoma associated with retention of fertility and normal pregnancies in females. Scotland and Newcastle Lymphoma Group, UK. Leuk Lymphoma 28 (1-2): 127-32, 1997. [PUBMED Abstract]
- Gangaraju R, Chen Y, Hageman L, et al.: Risk of venous thromboembolism in patients with non-Hodgkin lymphoma surviving blood or marrow transplantation. Cancer 125 (24): 4498-4508, 2019. [PUBMED Abstract]
- Westin JR, Thompson MA, Cataldo VD, et al.: Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk 13 (2): 99-105, 2013. [PUBMED Abstract]
Cellular Classification of Peripheral T-Cell Non-Hodgkin Lymphoma
A pathologist should be consulted before a biopsy because some studies require special preparation of tissue (e.g., frozen tissue). Knowledge of cell surface markers and immunoglobulin and T-cell receptor gene rearrangements may help with diagnostic and therapeutic decisions. The clonal excess of light-chain immunoglobulin may differentiate malignant cells from reactive cells. Because the prognosis and the approach to treatment are influenced by histopathology, outside biopsy specimens should be carefully reviewed by a hematopathologist who is experienced in diagnosing lymphomas. Although lymph node biopsies are recommended whenever possible, sometimes immunophenotypic data are sufficient for diagnosis of lymphoma when fine-needle aspiration cytology or core needle biopsy is preferred.[1,2]
Current Classification Systems
Updated REAL/WHO classification
The World Health Organization (WHO) modification of the Revised European American Lymphoma (REAL) classification recognizes three major categories of lymphoid malignancies based on morphology and cell lineage: B-cell neoplasms, T-cell/natural killer (NK)-cell neoplasms, and Hodgkin lymphoma (HL). Both lymphomas and lymphoid leukemias are included in this classification because both solid and circulating phases are present in many lymphoid neoplasms and distinction between them is artificial. For example, B-cell chronic lymphocytic leukemia (CLL) and B-cell small lymphocytic lymphoma are simply different manifestations of the same neoplasm, as are lymphoblastic lymphomas and acute lymphocytic leukemias. Within the B-cell and T-cell categories, two subdivisions are recognized: precursor neoplasms, which correspond to the earliest stages of differentiation, and more mature differentiated neoplasms.[3,4]
B-cell neoplasms
- Precursor B-cell neoplasm: precursor B-acute lymphoblastic leukemia/lymphoblastic lymphoma (LBL).
- Peripheral B-cell neoplasms.
- B-cell CLL/small lymphocytic lymphoma.
- B-cell prolymphocytic leukemia.
- Lymphoplasmacytic lymphoma/immunocytoma.
- Mantle cell lymphoma.
- Follicular lymphoma.
- Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphatic tissue (MALT) type.
- Nodal marginal zone B-cell lymphoma (± monocytoid B cells).
- Splenic marginal zone lymphoma (± villous lymphocytes).
- Hairy cell leukemia.
- Plasmacytoma/plasma cell myeloma.
- Diffuse large B-cell lymphoma.
- Burkitt lymphoma.
T-cell and putative NK-cell neoplasms
- Precursor T-cell neoplasm: precursor T-acute lymphoblastic leukemia/LBL. For more information, see Acute Lymphoblastic Leukemia Treatment.
- Peripheral T-cell and NK-cell neoplasms.
- T-cell CLL/prolymphocytic leukemia.
- T-cell granular lymphocytic leukemia.
- Mycosis fungoides (including Sézary syndrome).
- Peripheral T-cell lymphoma, not otherwise specified.
- Hepatosplenic gamma/delta T-cell lymphoma.
- Subcutaneous panniculitis-like T-cell lymphoma.
- Extranodal T-/NK-cell lymphoma, nasal type.
- Nodal lymphomas of T follicular helper cell origin (including angioimmunoblastic T-cell lymphoma, follicular peripheral T-cell lymphoma, and nodal peripheral T-cell lymphoma with T follicular helper phenotype).
- Enteropathy-associated intestinal T-cell lymphoma.
- Monomorphic epitheliotropic intestinal T-cell lymphoma.
- Adult T-cell lymphoma/leukemia (human T-lymphotrophic virus [HTLV] 1+).
- Anaplastic large cell lymphoma, primary systemic type.
- Anaplastic large cell lymphoma, primary cutaneous type.
- Aggressive NK-cell leukemia.
HL
- Nodular lymphocyte-predominant HL.
- Classical HL.
- Nodular sclerosis HL.
- Lymphocyte-rich classical HL.
- Mixed-cellularity HL.
- Lymphocyte-depleted HL.
The REAL classification encompasses all lymphoproliferative neoplasms. For more information, see the following PDQ summaries:
- Acute Lymphoblastic Leukemia Treatment
- Aggressive B-Cell Non-Hodgkin Lymphoma Treatment
- AIDS-Related Lymphoma Treatment
- Chronic Lymphocytic Leukemia Treatment
- Hodgkin Lymphoma Treatment
- Hairy Cell Leukemia Treatment
- Indolent B-Cell Non-Hodgkin Lymphoma Treatment
- Mantle Cell Lymphoma Treatment
- Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment
- Peripheral T-Cell Non-Hodgkin Lymphoma Treatment
- Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment
- Primary Central Nervous System Lymphoma Treatment
Subtypes of Peripheral T-Cell Non-Hodgkin Lymphoma
Peripheral T-cell non-Hodgkin lymphoma includes the following subtypes, among others:
- Anaplastic large cell lymphoma.
- Nodal lymphomas of T follicular helper cell origin (including angioimmunoblastic T-cell lymphoma, follicular peripheral T-cell lymphoma, and nodal peripheral T-cell lymphoma with T follicular helper phenotype).
- Peripheral T-cell lymphoma, not otherwise specified.
- Extranodal NK/T-cell lymphoma.
- Enteropathy-associated and monomorphic epitheliotropic intestinal T-cell lymphoma.
- Hepatosplenic T-cell lymphoma.
- Indolent T-cell lymphoma of the gastrointestinal tract.
- Adult T-cell leukemia/lymphoma.
- T-cell prolymphocytic leukemia.
- Cutaneous T-cell lymphoma (including mycosis fungoides and Sézary syndrome, subcutaneous panniculitis-like T-cell lymphoma, primary cutaneous anaplastic large cell lymphoma, primary cutaneous gamma-delta T-cell lymphoma, and others). For more information, see Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment.
- T-cell granular lymphocytic leukemia. For more information, see Chronic Lymphocytic Leukemia Treatment.
References
- Zeppa P, Marino G, Troncone G, et al.: Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-Hodgkin lymphoma: a critical review of 307 cases with technical suggestions. Cancer 102 (1): 55-65, 2004. [PUBMED Abstract]
- Young NA, Al-Saleem T: Diagnosis of lymphoma by fine-needle aspiration cytology using the revised European-American classification of lymphoid neoplasms. Cancer 87 (6): 325-45, 1999. [PUBMED Abstract]
- Pileri SA, Milani M, Fraternali-Orcioni G, et al.: From the R.E.A.L. Classification to the upcoming WHO scheme: a step toward universal categorization of lymphoma entities? Ann Oncol 9 (6): 607-12, 1998. [PUBMED Abstract]
- Society for Hematopathology Program: Society for Hematopathology Program. Am J Surg Pathol 21 (1): 114-121, 1997.
Stage Information for Peripheral T-Cell Non-Hodgkin Lymphoma
Stage is important in selecting a treatment for patients with non-Hodgkin lymphoma (NHL). Chest and abdominal computed tomography (CT) scans are usually part of the staging evaluation for all patients with lymphoma. The staging system for NHL is similar to the staging system used for Hodgkin lymphoma (HL).
It is common for patients with NHL to have involvement of the following sites:
- Noncontiguous lymph nodes.
- Waldeyer ring.
- Epitrochlear nodes.
- Gastrointestinal tract.
- Extranodal presentations. (A single extranodal site is occasionally the only site of involvement in patients with diffuse lymphoma.)
- Bone marrow.
- Liver (especially common in patients with low-grade lymphomas).
Cytological examination of cerebrospinal fluid may be positive in patients with aggressive NHL. Involvement of hilar and mediastinal lymph nodes is less common than in HL. Mediastinal adenopathy, however, is a prominent feature of lymphoblastic lymphoma and primary mediastinal B-cell lymphoma, entities primarily found in young adults.
Most patients with NHL present with advanced (stage III or stage IV) disease often identified by CT scans or biopsies of the bone marrow and other accessible sites of involvement. In a retrospective review of over 32,000 cases of lymphoma in France, up to 40% of diagnoses were made by core needle biopsy, and 60% were made by excisional biopsy.[1] After expert review, core needle biopsy provided a definite diagnosis in 92.3% of cases; excisional biopsy provided a definite diagnosis in 98.1% of cases (P < .0001). Laparoscopic biopsy or laparotomy is not required for staging but rarely may be necessary to establish a diagnosis or histological type.[2]
Positron emission tomography (PET) with fluorine F 18-fludeoxyglucose can be used for initial staging. It can also be used for follow-up after therapy as a supplement to CT scanning.[3] Multiple studies have demonstrated that interim PET scans after two to four cycles of therapy do not provide reliable prognostic information. A large cooperative group trial (ECOG-E344 [NCT00274924]) reported problems with interobserver reproducibility. Two prospective trials and one meta-analysis showed no differences in outcomes between PET-negative and PET-positive/biopsy-negative patients.[4-7]
In a retrospective study of 130 patients with diffuse large B-cell lymphoma, PET scanning identified all clinically important marrow involvement from lymphoma, and bone marrow biopsy did not upstage any patient's lymphoma.[8] A retrospective study of 580 patients with follicular lymphoma from seven National Cancer Institute–sponsored trials showed no improvement in assessing response to therapy when bone marrow biopsy was added to radiological imaging.[9] The workup of NHL should include bone marrow biopsy when management would change (e.g., determining limited stage vs. advanced stage) or when evaluating cytopenias.
Staging Subclassification System
Lugano classification
The American Joint Committee on Cancer (AJCC) has adopted the Lugano classification to evaluate and stage lymphoma.[10] The Lugano classification system replaces the Ann Arbor classification system, which was adopted in 1971 at the Ann Arbor Conference,[11] with some modifications 18 years later from the Cotswolds meeting.[12,13]
| Stage | Stage Description | Illustration |
|---|---|---|
| CSF = cerebrospinal fluid; CT = computed tomography; DLBCL = diffuse large B-cell lymphoma; NHL = non-Hodgkin lymphoma. | ||
| aHodgkin and Non-Hodgkin Lymphomas. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 937–58. | ||
| bStage II bulky may be considered either early or advanced stage based on lymphoma histology and prognostic factors. | ||
| cThe definition of disease bulk varies according to lymphoma histology. In the Lugano classification, bulk ln Hodgkin lymphoma is defined as a mass greater than one-third of the thoracic diameter on CT of the chest or a mass >10 cm. For NHL, the recommended definitions of bulk vary by lymphoma histology. In follicular lymphoma, 6 cm has been suggested based on the Follicular Lymphoma International Prognostic Index-2 and its validation. In DLBCL, cutoffs ranging from 5 cm to 10 cm have been used, although 10 cm is recommended. | ||
| Limited stage | ||
| I | Involvement of a single lymphatic site (i.e., nodal region, Waldeyer’s ring, thymus, or spleen). |
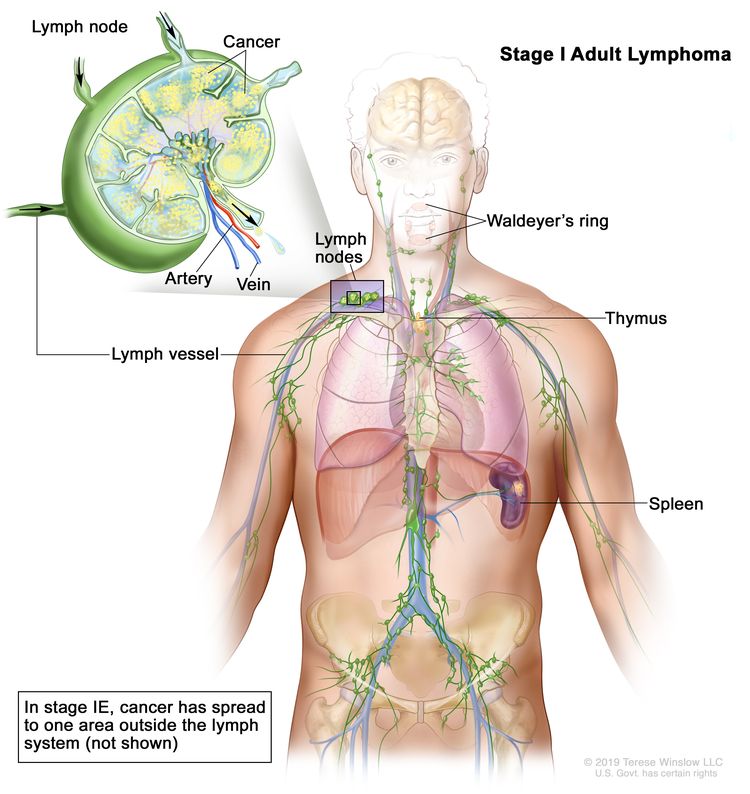 |
| IE | Single extralymphatic site in the absence of nodal involvement (rare in Hodgkin lymphoma). | |
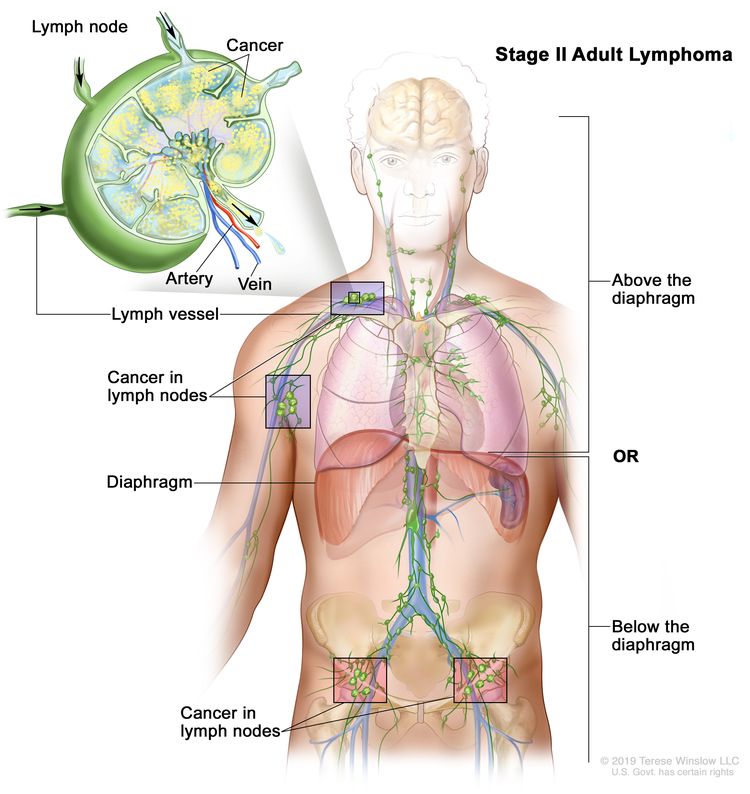
| II | Involvement of two or more lymph node regions on the same side of the diaphragm. |
 |
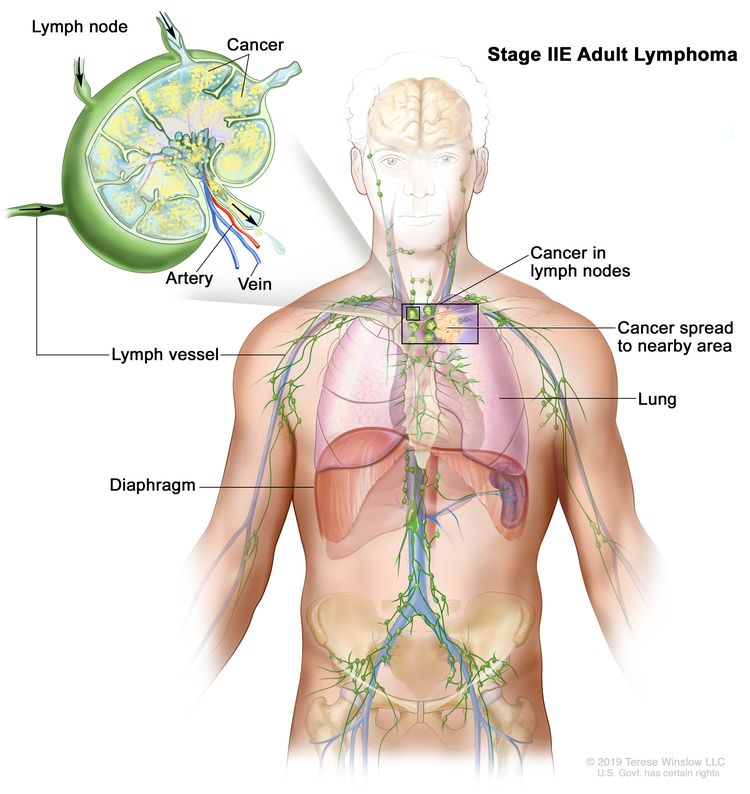
| IIE | Contiguous extralymphatic extension from a nodal site with or without involvement of other lymph node regions on the same side of the diaphragm. |
 |
| II bulkyb | Stage II with disease bulk.c | |
| Advanced stage | ||
| III | Involvement of lymph node regions on both sides of the diaphragm; nodes above the diaphragm with spleen involvement. |
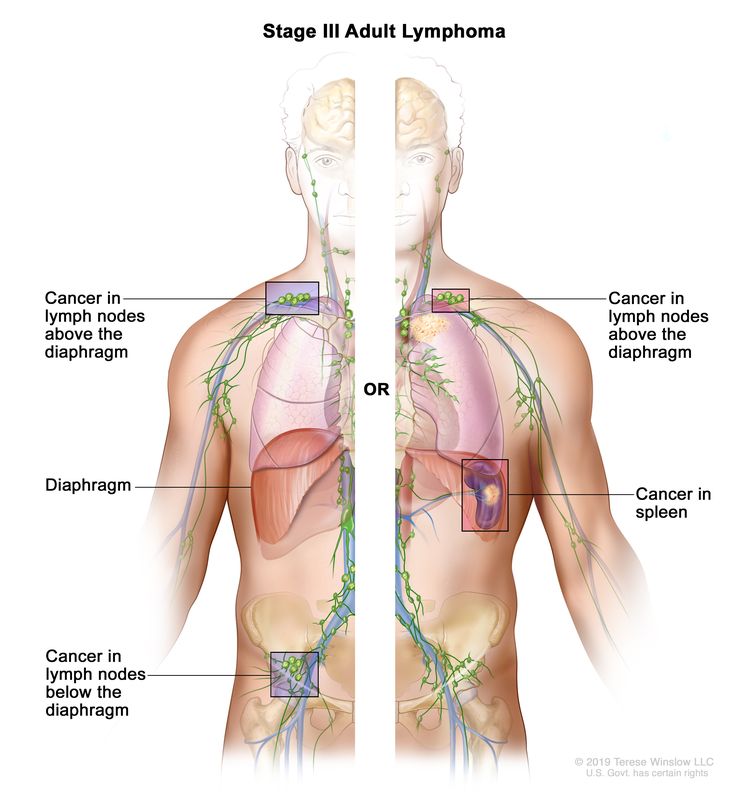 |
| IV | Diffuse or disseminated involvement of one or more extralymphatic organs, with or without associated lymph node involvement; or noncontiguous extralymphatic organ involvement in conjunction with nodal stage II disease; or any extralymphatic organ involvement in nodal stage III disease. Stage IV includes any involvement of the CSF, bone marrow, liver, or multiple lung lesions (other than by direct extension in stage IIE disease). |
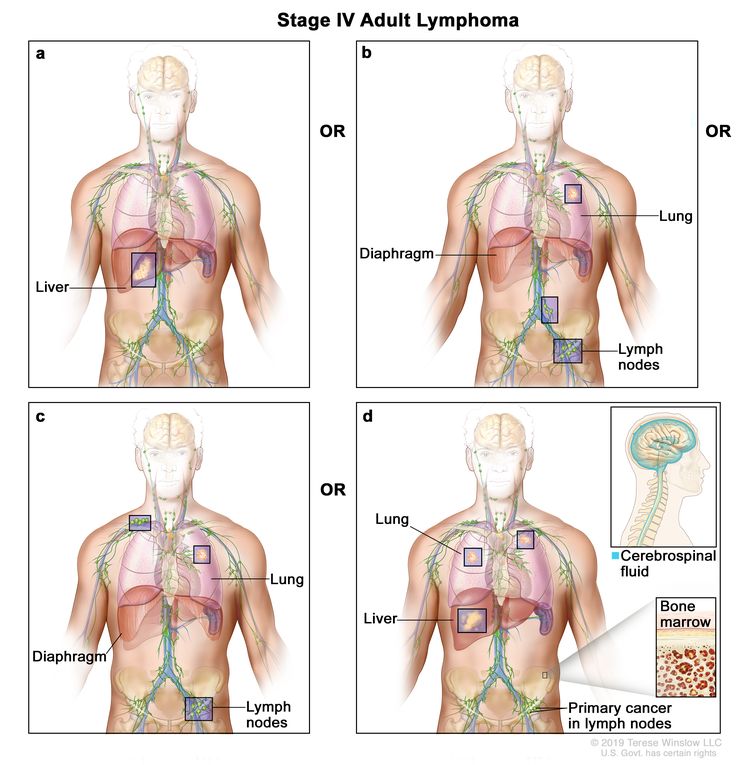 |
| Note: Hodgkin lymphoma uses A or B designation with stage group. A/B is no longer used in NHL. | ||
Occasionally, specialized staging systems are used. The physician should be aware of the system used in a specific report.
The E designation is used when extranodal lymphoid malignancies arise in tissues separate from, but near, the major lymphatic aggregates. Stage IV refers to disease that is diffusely spread throughout an extranodal site, such as the liver. If pathological proof of involvement of one or more extralymphatic sites has been documented, the symbol for the site of involvement, followed by a plus sign (+), is listed.
| N = nodes | H = liver | L = lung | M = bone marrow |
| S = spleen | P = pleura | O = bone | D = skin |
Current practice assigns a clinical stage based on the findings of the clinical evaluation and a pathological stage based on the findings from invasive procedures beyond the initial biopsy.
For example, on percutaneous biopsy, a patient with inguinal adenopathy and a positive lymphangiogram without systemic symptoms might have involvement of the liver and bone marrow. The precise stage of such a patient would be clinical stage IIA, pathological stage IVA(H+)(M+).
Several other factors that are not included in the above staging system are important for the staging and prognosis of patients with NHL. These factors include the following:
- Age.
- Performance status (PS).
- Tumor size.
- Lactate dehydrogenase (LDH) values.
- The number of extranodal sites.
The National Comprehensive Cancer Network International Prognostic Index (IPI) for aggressive NHL (diffuse large cell lymphoma) identifies the following five significant risk factors prognostic of overall survival (OS) and their associated risk scores:[14]
- Age.
- <40 years: 0.
- 41–60 years: 1.
- 61–75 years: 2.
- >75 years: 3.
- Stage III/IV: 1.
- Performance status (PS) 2/3/4: 1.
- Serum lactate dehydrogenase (LDH).
- Normalized: 0.
- >1x–3x: 1.
- >3x: 2.
- Number of extranodal sites ≥2: 1.
Risk scores:
- Low (0 or 1): 5-year OS rate, 96%; progression-free survival (PFS) rate, 91%.
- Low intermediate (2 or 3): 5-year OS rate, 82%; PFS rate, 74%.
- High intermediate (4 or 5): 5-year OS rate, 64%; PFS rate, 51%.
- High (>6): 5-year OS rate, 33%; PFS rate, 30%.
References
- Syrykh C, Chaouat C, Poullot E, et al.: Lymph node excisions provide more precise lymphoma diagnoses than core biopsies: a French Lymphopath network survey. Blood 140 (24): 2573-2583, 2022. [PUBMED Abstract]
- Mann GB, Conlon KC, LaQuaglia M, et al.: Emerging role of laparoscopy in the diagnosis of lymphoma. J Clin Oncol 16 (5): 1909-15, 1998. [PUBMED Abstract]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al.: Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32 (27): 3048-58, 2014. [PUBMED Abstract]
- Horning SJ, Juweid ME, Schöder H, et al.: Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood 115 (4): 775-7; quiz 918, 2010. [PUBMED Abstract]
- Moskowitz CH, Schöder H, Teruya-Feldstein J, et al.: Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol 28 (11): 1896-903, 2010. [PUBMED Abstract]
- Pregno P, Chiappella A, Bellò M, et al.: Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 119 (9): 2066-73, 2012. [PUBMED Abstract]
- Sun N, Zhao J, Qiao W, et al.: Predictive value of interim PET/CT in DLBCL treated with R-CHOP: meta-analysis. Biomed Res Int 2015: 648572, 2015. [PUBMED Abstract]
- Khan AB, Barrington SF, Mikhaeel NG, et al.: PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 122 (1): 61-7, 2013. [PUBMED Abstract]
- Rutherford SC, Yin J, Pederson L, et al.: Relevance of Bone Marrow Biopsies for Response Assessment in US National Cancer Institute National Clinical Trials Network Follicular Lymphoma Clinical Trials. J Clin Oncol 41 (2): 336-342, 2023. [PUBMED Abstract]
- Hodgkin and non-Hodgkin lymphoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 937–58.
- Carbone PP, Kaplan HS, Musshoff K, et al.: Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31 (11): 1860-1, 1971. [PUBMED Abstract]
- Lister TA, Crowther D, Sutcliffe SB, et al.: Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7 (11): 1630-6, 1989. [PUBMED Abstract]
- National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer 49 (10): 2112-35, 1982. [PUBMED Abstract]
- Zhou Z, Sehn LH, Rademaker AW, et al.: An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123 (6): 837-42, 2014. [PUBMED Abstract]
Treatment of Anaplastic Large Cell Lymphoma
Anaplastic large cell lymphoma (ALCL) is a peripheral T-cell lymphoma associated with the CD30 antigen. The translocation of chromosomes 2 and 5 creates a unique fusion protein with a nucleophosmin–anaplastic lymphoma kinase (ALK).[1,2] Patients whose lymphomas express ALK by immunohistochemistry are usually younger and may have systemic symptoms, extranodal disease, and advanced-stage disease. However, they have a more favorable survival rate than patients with ALK-negative disease.[3,4] ALK-negative ALCL has been further characterized by DUSP22 chromosomal rearrangements and the presence of TP63 pathogenic variants. While DUSP22 rearrangements are associated with improved prognosis, TP63 pathogenic variants are associated with poorer outcomes.[5]
Treatment Options for Anaplastic Large Cell Lymphoma
- A prospective randomized trial included 452 patients with CD30-positive T-cell lymphoma (CD30 expression >10%). Of these patients, 70% had ALCL (22% with ALK-positive disease and 48% with ALK-negative disease). The trial compared the previously used standard regimen, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), with brentuximab vedotin (an anti-CD30 monoclonal antibody conjugated to a cytotoxic agent) combined with cyclophosphamide, doxorubicin, and prednisone (A+CHP regimen).[6]
- With a median follow-up of 47.6 months, the 5-year overall survival (OS) rates were 70.1% (95% confidence interval [CI], 63.3%–75.9%) for patients who received A+CHP and 61.0% (95% CI, 54.0%–67.3%) for patients who received CHOP (hazard ratio [HR], 0.72; 95% CI, 0.53–0.99).[7][Level of evidence A1]
- The 5-year progression-free survival (PFS) rates were 51.4% (95% CI, 42.8%–59.4%) for patients who received A+CHP and 43.0% (95% CI, 35.8%–50.0%) for patients who received CHOP (HR, 0.70; 95% CI, 0.53–0.91).
- This established A+CHP as a new option for patients with ALCL or other CD30-positive T-cell lymphomas, such as angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, not otherwise specified.
- For patients with relapsed disease, anecdotal responses have been reported for brentuximab vedotin,[8-11] belinostat,[12] romidepsin,[13] and pralatrexate.[14][Level of evidence C3]
- In a phase II study (NCT00866047), 66% of 58 patients attained a complete response with brentuximab vedotin.[11]
- At a median follow-up of 58 months, the 5-year PFS rate was 57% (95% CI, 41%–74%), and the 5-year OS rate was 79% (95% CI, 65%–92%). Of the patients achieving a complete response, 42% underwent hematopoietic stem cell transplant (SCT).[11][Level of evidence C3]
- In a retrospective review, 39 patients with relapsed disease had a 3-year PFS rate of 50% after autologous or allogeneic SCT.[15][Level of evidence C2]
- A retrospective review of 84 patients with ALK-negative ALCL suggested a survival benefit with autologous SCT. This hypothesis requires confirmation in a randomized prospective trial.[16][Level of evidence C3]
- A retrospective study included 182 patients with relapsed or refractory ALCL (23% ALK-positive, 21% ALK-negative, and 56% ALK-unknown) who underwent allogeneic SCT.[17]
- The 5-year PFS rate was 41% (95% CI, 34%–49%), and the 5-year OS rate was 41% (95% CI, 49%–64%).[17][Level of evidence C3]
- On multivariate analysis, African American race (HR, 2.7; 95% CI, 1.6–4.8; P < .001) and refractory disease at time of allogeneic SCT (HR, 3.2; 95% CI, 1.6–6.2; P < .001) were predictive factors for inferior OS.
- Despite ALK positivity being a favorable prognostic factor, outcomes after allogeneic SCT in this study did not vary significantly according to ALK status.
ALCL in children is usually characterized by systemic and cutaneous disease and has high response rates and good OS with doxorubicin-based combination chemotherapy.[18] The ALK inhibitor crizotinib has been combined with chemotherapy for previously untreated pediatric patients, and crizotinib has been used to control disease in multiply relapsed pediatric patients.[19,20] Crizotinib is associated with a high risk (around 25%) of thromboembolism, especially pulmonary embolism, and prophylaxis is recommended. There are no reports supporting the use of crizotinib in adults.
Breast Implant–Associated Anaplastic Large Cell Lymphoma
Patients with breast implant–associated ALCL may do well without chemotherapy after capsulectomy and implant removal if the disease is confined to the fibrous capsule, and no associated mass or lymphadenopathy is present.[21-24] Most patients with breast implant–associated ALCL have a characteristic deletion at 20Q13.13 that may help to diagnostically distinguish it from cutaneous or systemic ALCL.[25]
Primary cutaneous ALCL is a distinct entity that is typically ALK-negative and has a very indolent/low-grade clinical course. Breast implant–associated ALCL almost always occurs with implants that have a textured surface (so-called macrotextured implants) that helps adhere to the breast. Warnings, suspensions, and bans of macrotextured implants have occurred worldwide. The risk of breast implant–associated ALCL is approximately 1 in 12,000 people, but this risk may rise with further follow-up.[26]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bai RY, Ouyang T, Miething C, et al.: Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood 96 (13): 4319-27, 2000. [PUBMED Abstract]
- Hapgood G, Savage KJ: The biology and management of systemic anaplastic large cell lymphoma. Blood 126 (1): 17-25, 2015. [PUBMED Abstract]
- Gascoyne RD, Aoun P, Wu D, et al.: Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 93 (11): 3913-21, 1999. [PUBMED Abstract]
- Sibon D, Fournier M, Brière J, et al.: Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol 30 (32): 3939-46, 2012. [PUBMED Abstract]
- Onaindia A, de Villambrosía SG, Prieto-Torres L, et al.: DUSP22-rearranged anaplastic lymphomas are characterized by specific morphological features and a lack of cytotoxic and JAK/STAT surrogate markers. Haematologica 104 (4): e158-e162, 2019. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 33 (3): 288-298, 2022. [PUBMED Abstract]
- Younes A, Bartlett NL, Leonard JP, et al.: Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363 (19): 1812-21, 2010. [PUBMED Abstract]
- Pro B, Advani R, Brice P, et al.: Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 30 (18): 2190-6, 2012. [PUBMED Abstract]
- Prince HM, Kim YH, Horwitz SM, et al.: Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 390 (10094): 555-566, 2017. [PUBMED Abstract]
- Pro B, Advani R, Brice P, et al.: Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 130 (25): 2709-2717, 2017. [PUBMED Abstract]
- O'Connor OA, Horwitz S, Masszi T, et al.: Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 33 (23): 2492-9, 2015. [PUBMED Abstract]
- Coiffier B, Pro B, Prince HM, et al.: Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30 (6): 631-6, 2012. [PUBMED Abstract]
- O'Connor OA, Horwitz S, Hamlin P, et al.: Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 27 (26): 4357-64, 2009. [PUBMED Abstract]
- Smith SM, Burns LJ, van Besien K, et al.: Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol 31 (25): 3100-9, 2013. [PUBMED Abstract]
- Brink M, Meeuwes FO, van der Poel MWM, et al.: Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood 140 (9): 1009-1019, 2022. [PUBMED Abstract]
- Furqan F, Ahn KW, Chen Y, et al.: Allogeneic haematopoietic cell transplant in patients with relapsed/refractory anaplastic large cell lymphoma. Br J Haematol 200 (1): 54-63, 2023. [PUBMED Abstract]
- Seidemann K, Tiemann M, Schrappe M, et al.: Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood 97 (12): 3699-706, 2001. [PUBMED Abstract]
- Lowe EJ, Reilly AF, Lim MS, et al.: Crizotinib in Combination With Chemotherapy for Pediatric Patients With ALK+ Anaplastic Large-Cell Lymphoma: The Results of Children's Oncology Group Trial ANHL12P1. J Clin Oncol 41 (11): 2043-2053, 2023. [PUBMED Abstract]
- Mossé YP, Voss SD, Lim MS, et al.: Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol 35 (28): 3215-3221, 2017. [PUBMED Abstract]
- Miranda RN, Aladily TN, Prince HM, et al.: Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 32 (2): 114-20, 2014. [PUBMED Abstract]
- Clemens MW, Medeiros LJ, Butler CE, et al.: Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 34 (2): 160-8, 2016. [PUBMED Abstract]
- Mehta-Shah N, Clemens MW, Horwitz SM: How I treat breast implant-associated anaplastic large cell lymphoma. Blood 132 (18): 1889-1898, 2018. [PUBMED Abstract]
- Jaffe ES, Ashar BS, Clemens MW, et al.: Best Practices Guideline for the Pathologic Diagnosis of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 38 (10): 1102-1111, 2020. [PUBMED Abstract]
- Los-de Vries GT, de Boer M, van Dijk E, et al.: Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood 136 (25): 2927-2932, 2020. [PUBMED Abstract]
- Kinslow CJ, Kim A, Sanchez GI, et al.: Incidence of Anaplastic Large-Cell Lymphoma of the Breast in the US, 2000 to 2018. JAMA Oncol 8 (9): 1354-1356, 2022. [PUBMED Abstract]
Treatment of Nodal Lymphomas of T Follicular Helper Cell Origin
The 2016 World Health Organization (WHO) classification recognized nodal lymphomas of T follicular helper (TFH) cell origin as a distinct subset of peripheral T-cell lymphoma (PTCL). Unified by a CD4+ TFH cell origin, this subset includes:[1]
- Angioimmunoblastic T-cell lymphoma (AITL or ATCL).
- Follicular peripheral T-cell lymphoma (F-PTCL).
- Nodal peripheral T-cell lymphoma with follicular helper phenotype (nodal PTCL with TFH phenotype).
AITL is the most common of these entities and is the second-most common subtype of PTCL.[2-5] Patients often present with profound lymphadenopathy, fever, night sweats, weight loss, skin rash, a positive Coombs test, and polyclonal hypergammaglobulinemia.[6] Opportunistic infections are frequent because of an underlying immune deficiency. B-cell Epstein-Barr virus genomes are detected in most affected patients.[7] For more information about weight loss, see Nutrition in Cancer Care and for more information about skin rash, see Pruritus.
The remaining subtypes of nodal lymphomas of TFH cell origin, including F-PTCL and nodal PTCL with TFH phenotype, are derived from the same cell of origin. However, unlike AITL, these subtypes are not associated with hypervascularity on nodal biopsy and often lack the hyperinflammatory symptoms characteristic of AITL.[1]
Treatment Options for Nodal Lymphomas of T Follicular Helper Cell Origin
Doxorubicin-based combination chemotherapy, such as the CHO(E)P regimen (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone), is commonly used for patients with AITL and other nodal lymphomas of TFH cell origin.[2,5] For CD30-positive cases, brentuximab vedotin combined with cyclophosphamide, doxorubicin, and prednisone is the proposed standard of care.[8][Level of evidence C3] Patients with AITL were included in a clinical trial involving mostly patients with anaplastic large cell lymphoma. A benefit for this smaller AITL subgroup cannot be established.[8,9][Level of evidence C3] For more information, see the Treatment of Anaplastic Large Cell Lymphoma section.
The International Peripheral T-Cell Lymphoma Project involving 22 international centers identified 243 patients with AITL. The 5-year overall survival rate was 33%, and the failure-free survival rate was 18%.[10] Myeloablative chemotherapy and radiation therapy with autologous or allogeneic peripheral stem cell support has been described in anecdotal reports.[11-16][Level of evidence C3] Anecdotal responses have been reported for patients who received cyclosporine,[17] pralatrexate,[18] bendamustine,[19] belinostat,[20] the histone deacetylase inhibitor romidepsin, hypomethylating agents (HMAs),[21,22] and brentuximab vedotin (even if there is little or no CD30 expression on the lymphoma).[23,24][Level of evidence C3] Occasional spontaneous remissions and protracted responses to steroids only have been reported. Given increased frequency of pathogenic variants in genes that regulate epigenetic modification—such as TET2, IDH2, and DNMT3A—there is increased interest in therapies targeting these aberrations with HMAs and histone deacetylase inhibitors with anecdotal reports of improved efficacy compared with other subsets of PTCL.[21,22,25][Level of evidence C3] Further studies are needed to better characterize the efficacy of this approach.[21,22,25]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Yoon SE, Cho J, Kim YJ, et al.: Comprehensive analysis of clinical, pathological, and genomic characteristics of follicular helper T-cell derived lymphomas. Exp Hematol Oncol 10 (1): 33, 2021. [PUBMED Abstract]
- Siegert W, Agthe A, Griesser H, et al.: Treatment of angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma using prednisone with or without the COPBLAM/IMVP-16 regimen. A multicenter study. Kiel Lymphoma Study Group. Ann Intern Med 117 (5): 364-70, 1992. [PUBMED Abstract]
- Jaffe ES: Angioimmunoblastic T-cell lymphoma: new insights, but the clinical challenge remains. Ann Oncol 6 (7): 631-2, 1995. [PUBMED Abstract]
- Siegert W, Nerl C, Agthe A, et al.: Angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol 6 (7): 659-64, 1995. [PUBMED Abstract]
- Lunning MA, Vose JM: Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood 129 (9): 1095-1102, 2017. [PUBMED Abstract]
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006. [PUBMED Abstract]
- Bräuninger A, Spieker T, Willenbrock K, et al.: Survival and clonal expansion of mutating "forbidden" (immunoglobulin receptor-deficient) epstein-barr virus-infected b cells in angioimmunoblastic t cell lymphoma. J Exp Med 194 (7): 927-40, 2001. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 33 (3): 288-298, 2022. [PUBMED Abstract]
- Federico M, Rudiger T, Bellei M, et al.: Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol 31 (2): 240-6, 2013. [PUBMED Abstract]
- Reimer P, Rüdiger T, Geissinger E, et al.: Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 27 (1): 106-13, 2009. [PUBMED Abstract]
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008. [PUBMED Abstract]
- Kyriakou C, Canals C, Finke J, et al.: Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 27 (24): 3951-8, 2009. [PUBMED Abstract]
- Park SI, Horwitz SM, Foss FM, et al.: The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 125 (9): 1507-1517, 2019. [PUBMED Abstract]
- Brink M, Meeuwes FO, van der Poel MWM, et al.: Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood 140 (9): 1009-1019, 2022. [PUBMED Abstract]
- Los-de Vries GT, de Boer M, van Dijk E, et al.: Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood 136 (25): 2927-2932, 2020. [PUBMED Abstract]
- Advani R, Horwitz S, Zelenetz A, et al.: Angioimmunoblastic T cell lymphoma: treatment experience with cyclosporine. Leuk Lymphoma 48 (3): 521-5, 2007. [PUBMED Abstract]
- Amengual JE, Lichtenstein R, Lue J, et al.: A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 131 (4): 397-407, 2018. [PUBMED Abstract]
- Damaj G, Gressin R, Bouabdallah K, et al.: Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol 31 (1): 104-10, 2013. [PUBMED Abstract]
- O'Connor OA, Horwitz S, Masszi T, et al.: Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 33 (23): 2492-9, 2015. [PUBMED Abstract]
- Wong J, Gruber E, Maher B, et al.: Integrated clinical and genomic evaluation of guadecitabine (SGI-110) in peripheral T-cell lymphoma. Leukemia 36 (6): 1654-1665, 2022. [PUBMED Abstract]
- Lemonnier F, Dupuis J, Sujobert P, et al.: Treatment with 5-azacytidine induces a sustained response in patients with angioimmunoblastic T-cell lymphoma. Blood 132 (21): 2305-2309, 2018. [PUBMED Abstract]
- Coiffier B, Pro B, Prince HM, et al.: Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30 (6): 631-6, 2012. [PUBMED Abstract]
- Fanale MA, Horwitz SM, Forero-Torres A, et al.: Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 131 (19): 2120-2124, 2018. [PUBMED Abstract]
- Pro B, Horwitz SM, Prince HM, et al.: Romidepsin induces durable responses in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma. Hematol Oncol 35 (4): 914-917, 2017. [PUBMED Abstract]
Treatment of Peripheral T-Cell Lymphoma, Not Otherwise Specified
Patients with peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) have diffuse large cell or diffuse mixed lymphoma that expresses a cell surface phenotype of a postthymic (or peripheral) T-cell expressing either CD4 or, less often, CD8.[1] PTCL-NOS encompasses a group of heterogeneous nodal T-cell lymphomas that will require future delineation.[2,3] Gene expression profiling studies have identified two distinct subtypes of PTCL-NOS based on the transcription factors GATA3 and TBX21. Early investigation and anecdotal reports suggest these subtypes may carry important prognostic implications and could predict response to certain therapies.[4] Additional studies are needed for further characterization of their clinical relevance.
Prognosis
Most investigators report worse response and survival rates for patients with PTCL-NOS than for patients with comparably staged B-cell aggressive lymphomas.[3,5] Most patients present with multiple adverse prognostic factors (i.e., older age, stage IV, multiple extranodal sites, and elevated lactate dehydrogenase), and these patients have low (<20%) failure-free survival and overall survival (OS) rates at 5 years.[3,5] As with other lymphomas (e.g., diffuse large B-cell lymphoma [DLBCL] or follicular lymphoma), event-free survival (EFS) at 24 months predicts a 5-year OS rate of 78%.[6]
Treatment Options for Peripheral T-Cell Lymphoma, Not Otherwise Specified
Therapy involves doxorubicin-based combination chemotherapy such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHO(E)P (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone). Doses are the same as those used for DLBCL.[7] For CD30-positive cases, brentuximab vedotin combined with cyclophosphamide, doxorubicin, and prednisone is the proposed standard of care.[8][Level of evidence C3] Patients with PTCL-NOS were included in the clinical trial involving mostly patients with anaplastic large cell lymphoma. A benefit for this smaller PTCL-NOS subgroup cannot be established.[8,9][Level of evidence C3] For more information, see the Treatment of Anaplastic Large Cell Lymphoma section.
For patients with early-stage disease, anecdotal retrospective series disagree on the value of consolidative radiation therapy after combination chemotherapy.[10][Level of evidence C3] Consolidation therapy using high-dose chemotherapy with autologous or allogeneic hematopoietic stem cell transplant (SCT) has been given to patients with advanced-stage PTCL after induction therapy in multiple phase II or retrospective trials. Evidence for this approach is anecdotal.[11-18][Level of evidence C3]
Evidence (CHOP, CHO[E]P, or other options for relapsing disease):
- A randomized prospective trial included 104 patients younger than 61 years with stage II, III, or IV PTCL (excluding ALK-positive anaplastic large cell lymphoma). Patients received either autologous SCT or allogeneic SCT as consolidation therapy after induction with CHO(E)P followed by DHAP (dexamethasone, cytarabine, and cisplatin).[19][Level of evidence C3]
- With a median follow-up of 42 months, the 3-year EFS rate was 43% for patients who received allogeneic SCT and 38% for patients who received autologous SCT.
- The 3-year OS rate was 57% for patients who received allogeneic SCT and 70% for patients who received autologous SCT (P = nonsignificant).
- None of the 21 responding patients who proceeded to allogeneic SCT relapsed, and 36% of patients who proceeded to autologous SCT relapsed.
- Eight of 26 patients (31%) who received allogeneic SCT died of graft-versus-host disease, and none of the 41 patients who received autologous SCT died of toxicity.
- The benefit of graft-versus-lymphoma effect was negated by increased transplant-related mortality.
- In a prospective trial of 109 evaluable patients with relapsing disease, treatment with pralatrexate resulted in a 30% response rate and a median 10-month duration of response.[20,21][Level of evidence C3]
- Similar response rates were seen in 130 evaluable patients with relapsing disease who received romidepsin in a prospective trial.[21][Level of evidence C3]
- Anecdotal responses have been seen with a combination of pralatrexate and romidepsin,[22] single-agent bendamustine,[23] belinostat,[24] and brentuximab vedotin (even if there is little or no CD30 expression on the lymphoma).[25][Level of evidence C3]
Incorporation of these new agents with CHOP chemotherapy is under clinical evaluation.[3,8]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Rüdiger T, Weisenburger DD, Anderson JR, et al.: Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol 13 (1): 140-9, 2002. [PUBMED Abstract]
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006. [PUBMED Abstract]
- Weisenburger DD, Savage KJ, Harris NL, et al.: Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 117 (12): 3402-8, 2011. [PUBMED Abstract]
- Amador C, Greiner TC, Heavican TB, et al.: Reproducing the molecular subclassification of peripheral T-cell lymphoma-NOS by immunohistochemistry. Blood 134 (24): 2159-2170, 2019. [PUBMED Abstract]
- Sonnen R, Schmidt WP, Müller-Hermelink HK, et al.: The International Prognostic Index determines the outcome of patients with nodal mature T-cell lymphomas. Br J Haematol 129 (3): 366-72, 2005. [PUBMED Abstract]
- Maurer MJ, Ellin F, Srour L, et al.: International Assessment of Event-Free Survival at 24 Months and Subsequent Survival in Peripheral T-Cell Lymphoma. J Clin Oncol 35 (36): 4019-4026, 2017. [PUBMED Abstract]
- Carson KR, Horwitz SM, Pinter-Brown LC, et al.: A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 123 (7): 1174-1183, 2017. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 33 (3): 288-298, 2022. [PUBMED Abstract]
- Briski R, Feldman AL, Bailey NG, et al.: Survival in patients with limited-stage peripheral T-cell lymphomas. Leuk Lymphoma 56 (6): 1665-70, 2015. [PUBMED Abstract]
- Rodriguez J, Munsell M, Yazji S, et al.: Impact of high-dose chemotherapy on peripheral T-cell lymphomas. J Clin Oncol 19 (17): 3766-70, 2001. [PUBMED Abstract]
- Reimer P, Rüdiger T, Geissinger E, et al.: Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 27 (1): 106-13, 2009. [PUBMED Abstract]
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008. [PUBMED Abstract]
- d'Amore F, Relander T, Lauritzsen GF, et al.: Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 30 (25): 3093-9, 2012. [PUBMED Abstract]
- Schmitz N, Lenz G, Stelljes M: Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood 132 (3): 245-253, 2018. [PUBMED Abstract]
- Park SI, Horwitz SM, Foss FM, et al.: The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 125 (9): 1507-1517, 2019. [PUBMED Abstract]
- Brink M, Meeuwes FO, van der Poel MWM, et al.: Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood 140 (9): 1009-1019, 2022. [PUBMED Abstract]
- Los-de Vries GT, de Boer M, van Dijk E, et al.: Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood 136 (25): 2927-2932, 2020. [PUBMED Abstract]
- Schmitz N, Truemper L, Bouabdallah K, et al.: A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood 137 (19): 2646-2656, 2021. [PUBMED Abstract]
- O'Connor OA, Pro B, Pinter-Brown L, et al.: Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 29 (9): 1182-9, 2011. [PUBMED Abstract]
- Coiffier B, Pro B, Prince HM, et al.: Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30 (6): 631-6, 2012. [PUBMED Abstract]
- Amengual JE, Lichtenstein R, Lue J, et al.: A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 131 (4): 397-407, 2018. [PUBMED Abstract]
- Damaj G, Gressin R, Bouabdallah K, et al.: Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol 31 (1): 104-10, 2013. [PUBMED Abstract]
- O'Connor OA, Horwitz S, Masszi T, et al.: Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 33 (23): 2492-9, 2015. [PUBMED Abstract]
- Fanale MA, Horwitz SM, Forero-Torres A, et al.: Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 131 (19): 2120-2124, 2018. [PUBMED Abstract]
Treatment of Extranodal Natural Killer/T-Cell Lymphoma
Extranodal natural killer (NK)/T-cell lymphoma, nasal type, is an aggressive lymphoma marked by extensive necrosis and angioinvasion, most often presenting in extranodal sites, in particular the nasal or paranasal sinus region.[1] Other extranodal sites include the palate, trachea, skin, and gastrointestinal tract. Hemophagocytic syndrome may also occur; historically, these tumors were considered part of lethal midline granuloma.[2] Tumor cells are typically NK cells with immunophenotyping showing CD3 and CD56 expression. In nearly all cases, Epstein-Barr virus (EBV) is detectable in the tumor cells. Aggressive NK cell leukemia (ANKL) is a separate but related diagnosis, which was previously recognized as a subset of large granular lymphocyte (LGL) leukemia, and is characterized by mature NK cell neoplastic involvement of the blood or marrow involvement with a particularly aggressive course and poor prognosis.[3] A benign NK-cell enteropathy (EBV negative) on endoscopic biopsy can be distinguished from NK/T-cell lymphoma.[3] Extranodal NK-cell lymphoma (ENKL), nasal type is rare in Western countries and more commonly seen in Asia, where it represents 3% to 8% of all lymphoma cases. Most cases present with nasal involvement and localized disease. Both extranasal and advanced-stage (stages III–IV) disease are associated with poor prognosis.[4,5]
Treatment Options for Extranodal Natural Killer/T-Cell Lymphoma
The management of localized ENKL with nasal involvement involves combined modality therapy with chemotherapy and radiation therapy for those fit for chemotherapy or radiation therapy alone for those unfit for chemotherapy. The management of nonlocalized nasal ENKL and all cases of extranasal ENKL involves combination chemotherapy with or without radiation therapy.[6] Because ENKL frequently expresses P-glycoprotein which confers multidrug resistance and reduces the efficacy of traditional anthracycline-based regimens, asparaginase is typically incorporated into the chemotherapy regimens.[7,8] Various asparaginase-based combination chemotherapy regimens have been used.
Evidence (asparaginase-based combination chemotherapy):
- A phase II study included 28 patients with newly diagnosed stage IV or relapsed or refractory ENKL. Patients received steroids, methotrexate, ifosfamide, [L-]asparaginase, and etoposide (SMILE).[9]
- The overall response rate after two cycles was 79% (95% confidence interval [CI], 65%–89%), and the complete response rate was 45%. The 1-year overall survival (OS) rate was 55% (95% CI, 38%–69%).
- Grade 4 neutropenia was observed in 92% of patients, and grade 3 or 4 infections were seen in 61% of patients.[9]
- A modification to the SMILE regimen (mSMILE) incorporating pegylated asparaginase has demonstrated reduced toxicity.[10]
Despite no head-to-head studies, this regimen is used more often in clinical practice, in patients with advanced-stage disease, or in combination with radiation therapy for patients with localized disease. Other asparaginase-based regimens which have been studied include gemcitabine, oxaliplatin, and pegaspargase (P-GEMOX);[10] gemcitabine, etoposide, pegaspargase, and dexamethasone (GELAD);[11] and dexamethasone, cisplatin, gemcitabine, and pegaspargase (DDGP).[12]For patients who may not tolerate or who have reactions to asparaginase, a nonasparaginase regimen may be given with concurrent radiation therapy.[13][Level of evidence C3]
- A retrospective study (NCT02733458) included 35 patients with newly diagnosed stage III to IV, relapsed, or refractory ENKL. Patients received P-GEMOX.[10]
- The overall response rate was 80.0%, and the complete response rate was 51.4%. The 3-year progression-free survival (PFS) rate was 38.6%, and the 3-year OS rate was 64.7%.[10][Level of evidence C3]
- A prospective multicenter study (NCT02733458) included 52 patients with newly diagnosed stage IE/IIE ENKL who received two cycles of GELAD followed by intensity-modulated radiotherapy (50–56 Gy in 25–58 fractions) and two additional courses of GELAD.[11]
- With a median follow-up of 32 months, the estimated 4-year OS rate was 94.2% (95% CI, 83.2%–93.1%), and the 4-year PFS rate was 90.4% (95% CI, 78.4%–95.9%).[11][Level of evidence C3]
- The overall response rate was 94.2%, and the complete response rate was 92.3%.
- A retrospective study included 80 patients with newly diagnosed (n = 48), refractory (n = 23), or first-relapse (n = 9) ENKL. Patients received DDGP.[12]
- The overall response rate was 91.3% (95% CI, 85.0%–96.3%), and the complete response rate was 60.0% (95% CI, 48.8%–71.3%). The 2-year PFS rate was 81.4% (95% CI, 76.3%–86.5%), and the 2-year OS rate was 87.1% (95% CI, 83.4%–91.4%).[12][Level of evidence C3]
- A phase I/II study of 27 patients with newly diagnosed stage IE or contiguous IIE disease studied the nonasparaginase-based regimen DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin). Patients received three cycles of DeVIC at the recommended phase II dosing and concurrent radiotherapy dosed at 50 Gy.[13]
- With a median follow-up of 32 months, the 2-year OS rate was 78% (95% CI, 57%–89%). The overall response rate was 81%, and the complete response rate was 77%.[13][Level of evidence C3]
- The most common grade 3 or higher nonhematologic toxicity was mucositis related to radiation, which occurred in 30% of patients.
Radiation therapy is an essential component of treatment for localized ENKL, nasal type, whether given as a monotherapy or as part of combined modality therapy.
Evidence (radiation therapy with or without chemotherapy):
- A retrospective review included 1,273 patients with early-stage disease. Patients were stratified into a low-risk group and high-risk group using stage, age, lactate dehydrogenase level, performance status, and primary tumor invasion.
- Low-risk patients fared best with radiation therapy alone,[14] while high-risk patients fared best with a strategy of radiation therapy combined with chemotherapy.[15-17][Level of evidence C3]
- A retrospective review included 303 previously untreated patients from an international consortium who received nonanthracycline chemotherapy.[18]
- The OS rates were identical (72%−74% at 5 years) for patients with early-stage disease who received either concurrent chemotherapy and radiation therapy or chemotherapy followed by radiation therapy.[18][Level of evidence C3]
Higher doses of radiation therapy administered at more than 50 Gy are associated with improved outcomes according to anecdotal reports.[17] The highly aggressive course, with poor response and short survival with standard therapies, especially for patients with advanced-stage disease or extranasal presentation, has led some investigators to recommend autologous or allogeneic peripheral stem cell transplant consolidation.[19-24][Level of evidence C3]
For patients with relapsed or refractory disease, therapies targeting programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1) have demonstrated promising results.
Evidence (immunotherapy):
- In a phase II trial, the anti–PD-L1 antibody avelumab was given to 21 patients with relapsed or refractory disease.[25]
- The complete response rate was 24%, and the overall response rate was 38%. The responses correlated with tumor PD-L1 expression.[25][Level of evidence C3]
- Treatment with pembrolizumab, an anti–PD-1 antibody, resulted in similar responses in patients with relapsed or refractory disease.[26][Level of evidence C3]
- A single-arm multicenter phase II study (GEMSTONE-201 [NCT03595657]) included 80 patients with relapsed or refractory ENKL. Patients received the anti–PD-L1 monoclonal antibody sugemalimab.
- The objective response rate was 44.9% (95% CI, 33.6%–56.6%), and the complete response rate was 35.9%. The 12-month duration of response rate was 82.5% (95% CI, 62.0%–92.6%).[27][Level of evidence B3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Tse E, Kwong YL: How I treat NK/T-cell lymphomas. Blood 121 (25): 4997-5005, 2013. [PUBMED Abstract]
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006. [PUBMED Abstract]
- Tang YT, Wang D, Luo H, et al.: Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer J 7 (12): 660, 2017. [PUBMED Abstract]
- Suzuki R, Suzumiya J, Yamaguchi M, et al.: Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol 21 (5): 1032-40, 2010. [PUBMED Abstract]
- Qi SN, Li YX, Specht L, et al.: Modern Radiation Therapy for Extranodal Nasal-Type NK/T-cell Lymphoma: Risk-Adapted Therapy, Target Volume, and Dose Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 110 (4): 1064-1081, 2021. [PUBMED Abstract]
- Obama K, Tara M, Niina K: L-asparaginase-Based induction therapy for advanced extranodal NK/T-cell lymphoma. Int J Hematol 78 (3): 248-50, 2003. [PUBMED Abstract]
- Ando M, Sugimoto K, Kitoh T, et al.: Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol 130 (6): 860-8, 2005. [PUBMED Abstract]
- Yamaguchi M, Kwong YL, Kim WS, et al.: Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 29 (33): 4410-6, 2011. [PUBMED Abstract]
- Ghione P, Qi S, Imber BS, et al.: Modified SMILE (mSMILE) and intensity-modulated radiotherapy (IMRT) for extranodal NK-T lymphoma nasal type in a single-center population. Leuk Lymphoma 61 (14): 3331-3341, 2020. [PUBMED Abstract]
- Wang JH, Wang L, Liu CC, et al.: Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget 7 (20): 29092-101, 2016. [PUBMED Abstract]
- Zhu Y, Tian S, Xu L, et al.: GELAD chemotherapy with sandwiched radiotherapy for patients with newly diagnosed stage IE/IIE natural killer/T-cell lymphoma: a prospective multicentre study. Br J Haematol 196 (4): 939-946, 2022. [PUBMED Abstract]
- Zhang L, Li S, Jia S, et al.: The DDGP (cisplatin, dexamethasone, gemcitabine, and pegaspargase) regimen for treatment of extranodal natural killer (NK)/T-cell lymphoma, nasal type. Oncotarget 7 (36): 58396-58404, 2016. [PUBMED Abstract]
- Yamaguchi M, Tobinai K, Oguchi M, et al.: Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 27 (33): 5594-600, 2009. [PUBMED Abstract]
- Yang Y, Cao JZ, Lan SM, et al.: Association of Improved Locoregional Control With Prolonged Survival in Early-Stage Extranodal Nasal-Type Natural Killer/T-Cell Lymphoma. JAMA Oncol 3 (1): 83-91, 2017. [PUBMED Abstract]
- Yang Y, Zhu Y, Cao JZ, et al.: Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood 126 (12): 1424-32; quiz 1517, 2015. [PUBMED Abstract]
- Yamaguchi M, Suzuki R, Oguchi M, et al.: Treatments and Outcomes of Patients With Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J Clin Oncol 35 (1): 32-39, 2017. [PUBMED Abstract]
- Vargo JA, Patel A, Glaser SM, et al.: The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer 123 (16): 3176-3185, 2017. [PUBMED Abstract]
- Kwong YL, Kim SJ, Tse E, et al.: Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol 29 (1): 256-263, 2018. [PUBMED Abstract]
- Liang R, Todd D, Chan TK, et al.: Treatment outcome and prognostic factors for primary nasal lymphoma. J Clin Oncol 13 (3): 666-70, 1995. [PUBMED Abstract]
- Cheung MM, Chan JK, Lau WH, et al.: Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 16 (1): 70-7, 1998. [PUBMED Abstract]
- Hausdorff J, Davis E, Long G, et al.: Non-Hodgkin's lymphoma of the paranasal sinuses: clinical and pathological features, and response to combined-modality therapy. Cancer J Sci Am 3 (5): 303-11, 1997 Sep-Oct. [PUBMED Abstract]
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008. [PUBMED Abstract]
- Au WY, Weisenburger DD, Intragumtornchai T, et al.: Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113 (17): 3931-7, 2009. [PUBMED Abstract]
- Yamaguchi M, Suzuki R, Oguchi M: Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 131 (23): 2528-2540, 2018. [PUBMED Abstract]
- Kim SJ, Lim JQ, Laurensia Y, et al.: Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood 136 (24): 2754-2763, 2020. [PUBMED Abstract]
- Kwong YL, Chan TSY, Tan D, et al.: PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 129 (17): 2437-2442, 2017. [PUBMED Abstract]
- Huang H, Tao R, Hao S, et al.: Sugemalimab Monotherapy for Patients With Relapsed or Refractory Extranodal Natural Killer/T-Cell Lymphoma (GEMSTONE-201): Results From a Single-Arm, Multicenter, Phase II Study. J Clin Oncol 41 (16): 3032-3041, 2023. [PUBMED Abstract]
Treatment of Enteropathy-Associated and Monomorphic Epitheliotropic Intestinal T-Cell Lymphomas
Enteropathy-associated T-cell lymphoma (EATL) and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) are rare subsets of peripheral T-cell lymphoma (PTCL) which together account for less than 5% of PTCL. EATL (sometimes referred to as type 1 EATL) is a complication of celiac disease and is more common in Europe. MEITL (sometimes referred to as type 2 EATL) is not associated with celiac disease and is more common in Asia. SETD2 pathogenic variants and, to a lesser extent, STAT5B pathogenic variants are seen in most cases of EATL and MEITL.[1,2] Because a gluten-free diet prevents the development of lymphoma in patients with celiac disease, patients diagnosed in childhood rarely develop EATL. The diagnosis of celiac disease is usually made by finding villous atrophy in the resected intestine. Surgery is often required for diagnosis and to avoid perforation during therapy.[3]
Treatment Options for Enteropathy-Associated and Monomorphic Epitheliotropic Intestinal T-Cell Lymphomas
Recommended management includes CHO(E)P (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone), but relapse is common and outcomes are generally poor.[4-6] Complications of treatment include gastrointestinal bleeding, small bowel perforation, and enterocolic fistulae. Patients often require parenteral nutrition. For more information on parenteral nutrition, see Nutrition in Cancer Care. Multifocal intestinal perforations and visceral abdominal involvement are seen at the time of relapse. Consolidation with either high-dose chemotherapy with autologous stem cell rescue or allogeneic stem cell transplant is often used in first or later remission.[4,7,8][Level of evidence C3] Evidence for this approach is anecdotal.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Veloza L, Cavalieri D, Missiaglia E, et al.: Monomorphic epitheliotropic intestinal T-cell lymphoma comprises morphologic and genomic heterogeneity impacting outcome. Haematologica 108 (1): 181-195, 2023. [PUBMED Abstract]
- Moffitt AB, Ondrejka SL, McKinney M, et al.: Enteropathy-associated T cell lymphoma subtypes are characterized by loss of function of SETD2. J Exp Med 214 (5): 1371-1386, 2017. [PUBMED Abstract]
- Egan LJ, Walsh SV, Stevens FM, et al.: Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol 21 (2): 123-9, 1995. [PUBMED Abstract]
- Gale J, Simmonds PD, Mead GM, et al.: Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol 18 (4): 795-803, 2000. [PUBMED Abstract]
- Daum S, Ullrich R, Heise W, et al.: Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol 21 (14): 2740-6, 2003. [PUBMED Abstract]
- Di Sabatino A, Biagi F, Gobbi PG, et al.: How I treat enteropathy-associated T-cell lymphoma. Blood 119 (11): 2458-68, 2012. [PUBMED Abstract]
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008. [PUBMED Abstract]
- Sieniawski M, Angamuthu N, Boyd K, et al.: Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 115 (18): 3664-70, 2010. [PUBMED Abstract]
Treatment of Indolent T-Cell Lymphoma of the Gastrointestinal Tract
In contrast to the more aggressive forms of T-cell lymphoma with intestinal involvement, indolent T-cell lymphoma of the gastrointestinal tract (iTCL-GI) often presents more indolently. iTCL-GI can be difficult to distinguish from inflammatory bowel disease or other autoimmune disorders with gastrointestinal involvement.[1,2]
iTCL-GI is often diagnosed when multiple biopsies from different sites show matching clones by T-cell receptor gene rearrangement testing. While the immunophenotype can vary (iTCL-GI is frequently CD4+ but can also be CD8+ or CD4-/CD8-), Ki-67 is typically very low at 5% to 10%. Unlike some other intestinal lymphomas, Epstein-Barr encoding region staining for Epstein-Barr virus is usually negative. Although initial reports of this rare entity suggested a low likelihood of transformation to a more aggressive process, transformation to peripheral T-cell lymphoma, not otherwise specific and other aggressive forms has since been documented.[3]
Treatment Options for Indolent T-Cell Lymphoma of the Gastrointestinal Tract
Response to multiagent chemotherapy is limited, and many patients can be managed with watchful waiting alone. Limited studies suggest that alterations in the JAK/STAT pathway or genes involved in epigenetic modification could serve as potential therapeutic targets.[4]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Margolskee E, Jobanputra V, Lewis SK, et al.: Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One 8 (7): e68343, 2013. [PUBMED Abstract]
- Perry AM, Warnke RA, Hu Q, et al.: Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 122 (22): 3599-606, 2013. [PUBMED Abstract]
- Sanguedolce F, Zanelli M, Zizzo M, et al.: Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review. Cancers (Basel) 13 (11): , 2021. [PUBMED Abstract]
- Sharma A, Oishi N, Boddicker RL, et al.: Recurrent STAT3-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood 131 (20): 2262-2266, 2018. [PUBMED Abstract]
Treatment of Hepatosplenic T-Cell Lymphoma
Hepatosplenic T-cell lymphoma (HSTCL) is a rare subtype of peripheral T-cell lymphoma (PTCL) comprising approximately 1% of PTCL. HSTCL often involves young men. HSTCL appears to be localized to the hepatic and splenic sinusoids, with cell surface expression of the gamma delta T-cell receptor.[1-3] Characteristic chromosomal abnormalities such as isochromosome 7q and trisomy 8 are also suggestive of the diagnosis.[4] While in most cases the neoplastic cells express a gamma delta T-cell receptor (hepatosplenic gamma delta T-cell lymphoma), there are reports of alpha beta T-cell receptor expression (hepatosplenic alpha beta T-cell lymphoma).[5] This lymphoma has an extremely poor prognosis and an extremely aggressive clinical course.
Treatment Options for Hepatosplenic T-Cell Lymphoma
HSTCL is treated with induction chemotherapy and stem cell transplant (SCT) consolidation.[3,6]
Evidence (induction chemotherapy and SCT consolidation):
- A meta-analysis of 118 patients with HSTCL compared CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like induction regimens.[7]
- Non–CHOP-based regimens (containing cytarabine, etoposide, and/or platinum-based treatment) were associated with improved outcomes, including an overall response rate of 82% versus 52% (P = .006) and a median overall survival (OS) of 36.5 months versus 18 months (P = .00014).
- Consolidation with allogeneic SCT was associated with an improved median OS of 33 months, versus 27 months (P = .016) for autologous SCT.[7][Level of evidence C3]
The use of ICE (ifosfamide, carboplatin, and etoposide) or IVAC (ifosfamide, etoposide, and high-dose cytarabine) has resulted in improved responses when compared with CHOP in other smaller studies as well.[8][Level of evidence D] Given the inadequate responses to CHOP or CHOP-like regimens, many clinicians are using ICE chemotherapy as front-line induction therapy, followed by consolidative allogeneic SCT for patients achieving a first remission. However, the efficacy of this approach is undetermined.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Belhadj K, Reyes F, Farcet JP, et al.: Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood 102 (13): 4261-9, 2003. [PUBMED Abstract]
- Chanan-Khan A, Islam T, Alam A, et al.: Long-term survival with allogeneic stem cell transplant and donor lymphocyte infusion following salvage therapy with anti-CD52 monoclonal antibody (Campath) in a patient with alpha/beta hepatosplenic T-cell non-Hodgkin's lymphoma. Leuk Lymphoma 45 (8): 1673-5, 2004. [PUBMED Abstract]
- Pro B, Allen P, Behdad A: Hepatosplenic T-cell lymphoma: a rare but challenging entity. Blood 136 (18): 2018-2026, 2020. [PUBMED Abstract]
- Travert M, Huang Y, de Leval L, et al.: Molecular features of hepatosplenic T-cell lymphoma unravels potential novel therapeutic targets. Blood 119 (24): 5795-806, 2012. [PUBMED Abstract]
- Macon WR, Levy NB, Kurtin PJ, et al.: Hepatosplenic alphabeta T-cell lymphomas: a report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am J Surg Pathol 25 (3): 285-96, 2001. [PUBMED Abstract]
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008. [PUBMED Abstract]
- Klebaner D, Koura D, Tzachanis D, et al.: Intensive Induction Therapy Compared With CHOP for Hepatosplenic T-cell Lymphoma. Clin Lymphoma Myeloma Leuk 20 (7): 431-437.e2, 2020. [PUBMED Abstract]
- Voss MH, Lunning MA, Maragulia JC, et al.: Intensive induction chemotherapy followed by early high-dose therapy and hematopoietic stem cell transplantation results in improved outcome for patients with hepatosplenic T-cell lymphoma: a single institution experience. Clin Lymphoma Myeloma Leuk 13 (1): 8-14, 2013. [PUBMED Abstract]
Treatment of Adult T-Cell Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma (ATL) is caused by infection with the retrovirus human T-lymphotrophic virus 1 (HTLV1) and is frequently associated with lymphadenopathy, hypercalcemia, circulating leukemic cells, bone and skin involvement, hepatosplenomegaly, a rapidly progressive course, and poor response to combination chemotherapy.[1,2] HTLV1 is endemic to Japan (especially the southern island of Kyushu), central Africa, the Caribbean islands, and some regions of Central and South America, the Middle East, and Australia. This retrovirus is mostly spread by sexual contact or breastfeeding.[3] ATL has been divided into four clinical subtypes:[3]
- Acute (aggressive course with leukemia, with or without extranodal or nodal involvement).
- Lymphoma (aggressive course with lymphadenopathy and no leukemia).
- Chronic (indolent course with leukemia and lymphadenopathy).
- Smoldering (indolent course with only leukemia).
Treatment Options for Adult T-Cell Leukemia/Lymphoma
Treatment options for ATL include:
Biological and targeted therapies
Mogamulizumab
The anti-CCR4 monoclonal antibody mogamulizumab has a targeted lysis of the ATL clone and also reduces the immunosuppressive Treg population, allowing improvement of cell-mediated immunotherapy.[4] Single-agent phase II trials in patients with ATL showed response rates ranging from 30% to 50%.[5-7][Level of evidence C3] In a phase II study that combined mogamulizumab with combination chemotherapy, 101 patients had an overall response rate of 65% (95% confidence interval [CI], 55%–75%), a median progression-free survival of 7.4 months (95% CI, 5.7–9.1), and a median overall survival (OS) of 16 months (95% CI, 10.3–21.8).[8][Level of evidence C3]
A retrospective review evaluated mogamulizumab combined with both the VCAP-AMP-VECP (vincristine, cyclophosphamide, doxorubicin, prednisone; doxorubicin, ranimustine, prednisone; vindesine, etoposide, carboplatin, prednisone) regimen and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-based regimens versus chemotherapy alone. The review concluded that the 4-year OS rate was 46.3% in the mogamulizumab combination groups, compared with 20.6% in the chemotherapy-alone group.[9][Level of evidence C3] The best results were seen with CHOP-based regimens. Mogamulizumab is available in the United States but has not been approved by the U.S. Food and Drug Administration (FDA) for patients with ATL.
Mogamulizumab is often avoided in patients planning allogeneic SCT based on data from Japan that showed an increased risk of severe graft-versus-host disease (GVHD) in patients who received mogamulizumab before allogeneic SCT.[10] The relevance of these findings in other countries and the impact of different GVHD prophylaxis regimens in this setting remains to be determined.
Brentuximab vedotin
Brentuximab vedotin is a monoclonal antibody-drug conjugate directed at CD30 and is a treatment option for patients with ATL whose tumor expresses CD30.[11] Patients with ATL were included in a clinical trial of brentuximab vedotin that mostly involved patients with anaplastic large T-cell lymphoma. A statistical benefit for the very small ATL subgroup could not be established.[11][Level of evidence C3]
Lenalidomide
In a multicenter phase II study (NCT01724177) of 26 patients with relapsed ATL, the overall response rate was 42% (95% CI, 23%–63%) in patients who received the immunomodulatory agent lenalidomide.[12][Level of evidence C3]
Valemetostat
Valemetostat is a selective dual inhibitor of EZH1 and EZH2, which are important histone methyltransferases involved with chromatin folding.[13] A multicenter phase II study (NCT04102150) enrolled 25 patients with relapsed or refractory ATL, 24 of whom had prior therapy with mogamulizumab.[14] With a median follow-up of 6.5 months, the overall response rate was 48% (90% CI, 30.5%–65.9%). There were five complete remissions and seven partial remissions among the heavily pretreated patients.[14][Level of evidence C3] Responses were seen in patients with the acute, lymphoma, and chronic versions of ATL. Although approved for use in Japan, valemetostat is not approved by the FDA for use in the United States.
Zidovudine and interferon alfa
The combination of zidovudine and interferon alfa has activity against ATL, especially among patients with the chronic and smoldering versions of ATL.[15,16] Durable remissions of 8 to 12 months and response rates of 20% to 40% have been reported for patients, some of whom had relapsed disease after prior chemotherapy,[15,16] and some with the acute and lymphoma subtypes of ATL.[17,18]
Cytotoxic chemotherapy and allogeneic SCT
The acute and lymphoma types of ATL respond poorly to combination cytotoxic chemotherapy and allogeneic SCT, with a median OS under 1 year.[19-21] Less than 10% of patients who received combination therapy were alive after 4 years in one retrospective study.[21] Durable remissions have been reported after allogeneic SCT and even after subsequent donor lymphocyte infusion for relapses after transplant.[22-24][Level of evidence C3] Among 815 patients who underwent allogeneic SCT in two retrospective reviews, the 3-year OS rates were 36% and 26%.[25,26][Level of evidence C1]
The cytotoxic chemotherapy backbone for patients with ATL in the United States and Europe is the CHOP or CHO(E)P regimen (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone). Brentuximab vedotin has been added to CHOP in rare cases when patients express CD30. For the acute and lymphoma versions of ATL, a series of clinical trials in Japan established VCAP-AMP-VECP as the standard first-line treatment.[2,27,28] This regimen has generally not been implemented outside of Asia since a trial comparing VCAP-AMP-VECP versus CHOP-14 did not show statistically improved OS (3-year OS rate, 24% vs. 13%; P = nonsignificant).
Allogeneic SCT is frequently used for patients achieving first or later remission, although results are largely anecdotal.[29,30]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bangham CRM: HTLV-1 persistence and the oncogenesis of adult T-cell leukemia/lymphoma. Blood 141 (19): 2299-2306, 2023. [PUBMED Abstract]
- Cook LB, Phillips AA: How I treat adult T-cell leukemia/lymphoma. Blood 137 (4): 459-470, 2021. [PUBMED Abstract]
- Mehta-Shah N, Ratner L, Horwitz SM: Adult T-Cell Leukemia/Lymphoma. J Oncol Pract 13 (8): 487-492, 2017. [PUBMED Abstract]
- Sugata K, Yasunaga J, Miura M, et al.: Enhancement of anti-STLV-1/HTLV-1 immune responses through multimodal effects of anti-CCR4 antibody. Sci Rep 6: 27150, 2016. [PUBMED Abstract]
- Ishida T, Joh T, Uike N, et al.: Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 30 (8): 837-42, 2012. [PUBMED Abstract]
- Phillips AA, Fields PA, Hermine O, et al.: Mogamulizumab versus investigator's choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica 104 (5): 993-1003, 2019. [PUBMED Abstract]
- Ureshino H, Kamachi K, Kimura S: Mogamulizumab for the Treatment of Adult T-cell Leukemia/Lymphoma. Clin Lymphoma Myeloma Leuk 19 (6): 326-331, 2019. [PUBMED Abstract]
- Yonekura K, Kusumoto S, Choi I, et al.: Mogamulizumab for adult T-cell leukemia-lymphoma: a multicenter prospective observational study. Blood Adv 4 (20): 5133-5145, 2020. [PUBMED Abstract]
- Shichijo T, Nosaka K, Tatetsu H, et al.: Beneficial impact of first-line mogamulizumab-containing chemotherapy in adult T-cell leukaemia-lymphoma. Br J Haematol 198 (6): 983-987, 2022. [PUBMED Abstract]
- Sugio T, Kato K, Aoki T, et al.: Mogamulizumab Treatment Prior to Allogeneic Hematopoietic Stem Cell Transplantation Induces Severe Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant 22 (9): 1608-1614, 2016. [PUBMED Abstract]
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019. [PUBMED Abstract]
- Ishida T, Fujiwara H, Nosaka K, et al.: Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J Clin Oncol 34 (34): 4086-4093, 2016. [PUBMED Abstract]
- Chihara D: Synthetic lethality in ATL. Blood 141 (10): 1096-1098, 2023. [PUBMED Abstract]
- Izutsu K, Makita S, Nosaka K, et al.: An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma. Blood 141 (10): 1159-1168, 2023. [PUBMED Abstract]
- Bazarbachi A, Plumelle Y, Carlos Ramos J, et al.: Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 28 (27): 4177-83, 2010. [PUBMED Abstract]
- Bazarbachi A, Suarez F, Fields P, et al.: How I treat adult T-cell leukemia/lymphoma. Blood 118 (7): 1736-45, 2011. [PUBMED Abstract]
- Cook LB, Rowan AG, Demontis MA, et al.: Long-term clinical remission maintained after cessation of zidovudine and interferon-α therapy in chronic adult T-cell leukemia/lymphoma. Int J Hematol 107 (3): 378-382, 2018. [PUBMED Abstract]
- Hodson A, Crichton S, Montoto S, et al.: Use of zidovudine and interferon alfa with chemotherapy improves survival in both acute and lymphoma subtypes of adult T-cell leukemia/lymphoma. J Clin Oncol 29 (35): 4696-701, 2011. [PUBMED Abstract]
- Yamada Y, Tomonaga M, Fukuda H, et al.: A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol 113 (2): 375-82, 2001. [PUBMED Abstract]
- Fukushima T, Miyazaki Y, Honda S, et al.: Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 19 (5): 829-34, 2005. [PUBMED Abstract]
- Katsuya H, Yamanaka T, Ishitsuka K, et al.: Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J Clin Oncol 30 (14): 1635-40, 2012. [PUBMED Abstract]
- Itonaga H, Tsushima H, Taguchi J, et al.: Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: the Nagasaki Transplant Group experience. Blood 121 (1): 219-25, 2013. [PUBMED Abstract]
- Cook LB, Fuji S, Hermine O, et al.: Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J Clin Oncol 37 (8): 677-687, 2019. [PUBMED Abstract]
- Yoshimitsu M, Tanosaki R, Kato K, et al.: Risk Assessment in Adult T Cell Leukemia/Lymphoma Treated with Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 24 (4): 832-839, 2018. [PUBMED Abstract]
- Ishida T, Hishizawa M, Kato K, et al.: Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 120 (8): 1734-41, 2012. [PUBMED Abstract]
- Katsuya H, Ishitsuka K, Utsunomiya A, et al.: Treatment and survival among 1594 patients with ATL. Blood 126 (24): 2570-7, 2015. [PUBMED Abstract]
- Hermine O, Ramos JC, Tobinai K: A Review of New Findings in Adult T-cell Leukemia-Lymphoma: A Focus on Current and Emerging Treatment Strategies. Adv Ther 35 (2): 135-152, 2018. [PUBMED Abstract]
- Tsukasaki K, Utsunomiya A, Fukuda H, et al.: VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 25 (34): 5458-64, 2007. [PUBMED Abstract]
- Bazarbachi AH, Reef D, Narvel H, et al.: Outcome of Stem Cell Transplantation in HTLV-1-Associated North American Adult T-Cell Leukemia/Lymphoma. Clin Hematol Int 5 (2-3): 78-91, 2023. [PUBMED Abstract]
- Tanase AD, Colita A, Craciun OG, et al.: Allogeneic Stem Cell Transplantation for Adult T-Cell Leukemia/Lymphoma-Romanian Experience. J Clin Med 9 (8): , 2020. [PUBMED Abstract]
Treatment of T-Cell Prolymphocytic Leukemia
Prolymphocytic leukemia (PLL) is a rare form of lymphocytic leukemia characterized by excessive prolymphocytes in the blood with a typical phenotype that is positive for CD19, CD20, and surface-membrane immunoglobulin and negative for CD5.[1] These patients demonstrate splenomegaly and poor response to low-dose or high-dose chemotherapy.[2,3]
Treatment Options for T-Cell Prolymphocytic Leukemia
Cladribine appears to be an active agent (60% complete remission rate) for patients with de novo B-cell PLL.[4][Level of evidence C3] Anecdotal responses have been seen with venetoclax.[5][Level of evidence C3] Alemtuzumab, an anti-CD52 humanized monoclonal antibody, has been used for 76 patients with T-cell PLL after failure of previous chemotherapy (usually pentostatin or cladribine). The response rate was 51% (95% confidence interval, 40%–63%), and the median time to progression was 4.5 months (range, 0.1–45.4).[6][Level of evidence C3] These response rates have been confirmed by other investigators.[7] Patients with chronic lymphocytic leukemia (CLL) and prolymphocytoid transformation maintain the classic CLL phenotype and have a worse prognosis than patients with PLL.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Staber PB, Herling M, Bellido M, et al.: Consensus criteria for diagnosis, staging, and treatment response assessment of T-cell prolymphocytic leukemia. Blood 134 (14): 1132-1143, 2019. [PUBMED Abstract]
- Rozman C, Montserrat E: Chronic lymphocytic leukemia. N Engl J Med 333 (16): 1052-7, 1995. [PUBMED Abstract]
- Melo JV, Catovsky D, Galton DA: The relationship between chronic lymphocytic leukaemia and prolymphocytic leukaemia. I. Clinical and laboratory features of 300 patients and characterization of an intermediate group. Br J Haematol 63 (2): 377-87, 1986. [PUBMED Abstract]
- Saven A, Lee T, Schlutz M, et al.: Major activity of cladribine in patients with de novo B-cell prolymphocytic leukemia. J Clin Oncol 15 (1): 37-43, 1997. [PUBMED Abstract]
- Boidol B, Kornauth C, van der Kouwe E, et al.: First-in-human response of BCL-2 inhibitor venetoclax in T-cell prolymphocytic leukemia. Blood 130 (23): 2499-2503, 2017. [PUBMED Abstract]
- Keating MJ, Cazin B, Coutré S, et al.: Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol 20 (1): 205-13, 2002. [PUBMED Abstract]
- Dearden CE, Matutes E, Catovsky D: Alemtuzumab in T-cell malignancies. Med Oncol 19 (Suppl): S27-32, 2002. [PUBMED Abstract]
Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma
Treatment Options for Relapsed or Refractory Peripheral T-Cell Lymphoma
Treatment options for relapsed or refractory peripheral T-cell lymphoma include:
- Combination chemotherapy.
- ICE (ifosfamide, carboplatin, and etoposide).[1,2]
- GEMOX (gemcitabine, oxaliplatin, and dexamethasone).[3]
- DHAP (dexamethasone, high-dose cytarabine, and cisplatin).[4]
- ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin).[5,6]
- Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone).[7]
- Antibody conjugates.
- Brentuximab vedotin (for CD30-positive patients).
- Histone deacetylase inhibitors.
- Dihydrofolate reductase inhibitors.
- Pralatrexate.[11]
- Anti-CD52 monoclonal antibodies.
- Alemtuzumab (only available through a restricted distribution program).
- Stem cell transplant.[12-16]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Jerkeman M, Leppä S, Kvaløy S, et al.: ICE (ifosfamide, carboplatin, etoposide) as second-line chemotherapy in relapsed or primary progressive aggressive lymphoma--the Nordic Lymphoma Group experience. Eur J Haematol 73 (3): 179-82, 2004. [PUBMED Abstract]
- Zelenetz AD, Hamlin P, Kewalramani T, et al.: Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin's lymphoma. Ann Oncol 14 (Suppl 1): i5-10, 2003. [PUBMED Abstract]
- Shen QD, Wang L, Zhu HY, et al.: Gemcitabine, oxaliplatin and dexamethasone (GemDOx) as salvage therapy for relapsed or refractory diffuse large B-cell lymphoma and peripheral T-cell lymphoma. J Cancer 12 (1): 163-169, 2021. [PUBMED Abstract]
- Schmitz N, Truemper L, Bouabdallah K, et al.: A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood 137 (19): 2646-2656, 2021. [PUBMED Abstract]
- Velasquez WS, McLaughlin P, Tucker S, et al.: ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol 12 (6): 1169-76, 1994. [PUBMED Abstract]
- Mercadal S, Briones J, Xicoy B, et al.: Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol 19 (5): 958-63, 2008. [PUBMED Abstract]
- Hapgood G, Stone JM, Zannino D, et al.: A phase II study of a modified hyper-CVAD frontline therapy for patients with adverse risk diffuse large B-cell and peripheral T-cell non-Hodgkin lymphoma. Leuk Lymphoma 60 (4): 904-911, 2019. [PUBMED Abstract]
- Iyer SP, Foss FF: Romidepsin for the Treatment of Peripheral T-Cell Lymphoma. Oncologist 20 (9): 1084-91, 2015. [PUBMED Abstract]
- Campbell P, Thomas CM: Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma. J Oncol Pharm Pract 23 (2): 143-147, 2017. [PUBMED Abstract]
- O'Connor OA, Horwitz S, Masszi T, et al.: Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 33 (23): 2492-9, 2015. [PUBMED Abstract]
- O'Connor OA, Pro B, Pinter-Brown L, et al.: Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 29 (9): 1182-9, 2011. [PUBMED Abstract]
- Schmitz N, Lenz G, Stelljes M: Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood 132 (3): 245-253, 2018. [PUBMED Abstract]
- Chen AI, McMillan A, Negrin RS, et al.: Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant 14 (7): 741-7, 2008. [PUBMED Abstract]
- Hamadani M, Ngoya M, Sureda A, et al.: Outcome of allogeneic transplantation for mature T-cell lymphomas: impact of donor source and disease characteristics. Blood Adv 6 (3): 920-930, 2022. [PUBMED Abstract]
- Mehta-Shah N, Kommalapati A, Teja S: Successful treatment of mature T-cell lymphoma with allogeneic stem cell transplantation: the largest multicenter retrospective analysis. [Abstract] Blood 136 (Suppl 1): A-624, 35-36, 2020.
- Sterling CH, Hughes MS, Tsai HL, et al.: Allogeneic Blood or Marrow Transplantation with Post-Transplantation Cyclophosphamide for Peripheral T Cell Lymphoma: The Importance of Graft Source. Transplant Cell Ther 29 (4): 267.e1-267.e5, 2023. [PUBMED Abstract]
Latest Updates to This Summary (05/13/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult peripheral T-cell non-Hodgkin lymphoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Peripheral T-Cell Non-Hodgkin Lymphoma Treatment are:
- Eric J. Seifter, MD (Johns Hopkins University)
- Cole H. Sterling, MD (Johns Hopkins University)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Peripheral T-Cell Non-Hodgkin Lymphoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lymphoma/hp/peripheral-t-cell-lymphoma-pdq. Accessed <MM/DD/YYYY>. [PMID: 37437079]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.