Childhood Tracheobronchial Tumors Treatment (PDQ®)–Health Professional Version
Types of Childhood Tracheobronchial Tumors
Primary lung tumors are rare in children and histologically quite diverse.[1] A review of primary malignant epithelial lung tumors using the National Cancer Database found that the most common primary malignant pediatric lung neoplasms were carcinoid tumors (63%), followed by mucoepidermoid carcinoma of the lung (18%).[2]
Tracheobronchial tumors are a heterogeneous group of primary endobronchial lesions. Although adenoma implies a benign process, all varieties of tracheobronchial tumors occasionally display malignant behavior. Most primary lung tumors are malignant. In a review of 383 primary pulmonary neoplasms in children, 76% were malignant and 24% were benign.[3]
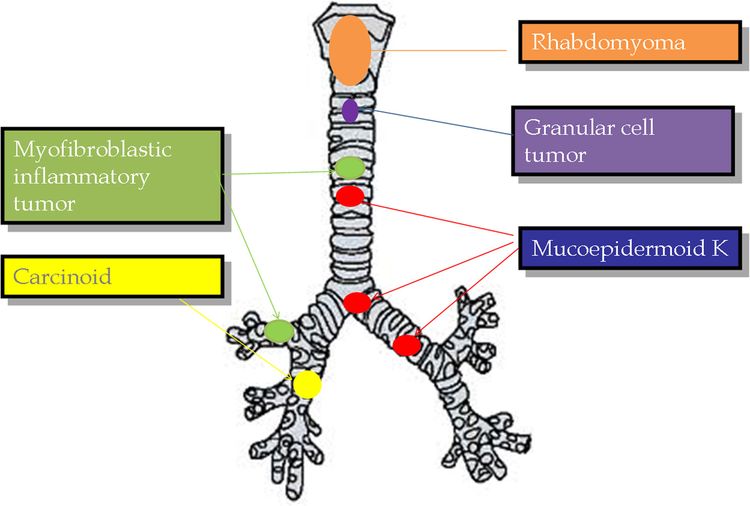
The following histological types have been identified (see Figure 1):[4-10]
- Carcinoid tumors (neuroendocrine tumors of the bronchus). Carcinoid tumors account for 80% to 85% of all tracheobronchial tumors in children.[4-8]; [11][Level of evidence C1] They are the most common tracheobronchial tumor.
- Mucoepidermoid carcinomas. Slow-growing vascular polypoid masses of the airway that are the second most common (10%) pediatric tracheobronchial tumor.
- Inflammatory myofibroblastic tumors. These low-grade tumors account for 1% of pediatric tracheobronchial tumors, are commonly located in the upper trachea, and rarely metastasize. Intrabronchial (pulmonary) inflammatory myofibroblastic tumors are incredibly rare compared with other sites of disease. For more information, see Childhood Pulmonary Inflammatory Myofibroblastic Tumors Treatment.
- Rhabdomyosarcomas.
- Granular cell tumors. Malignant transformation of these tumors has not been documented in pediatric patients.
References
- Yu DC, Grabowski MJ, Kozakewich HP, et al.: Primary lung tumors in children and adolescents: a 90-year experience. J Pediatr Surg 45 (6): 1090-5, 2010. [PUBMED Abstract]
- Rojas Y, Shi YX, Zhang W, et al.: Primary malignant pulmonary tumors in children: a review of the national cancer data base. J Pediatr Surg 50 (6): 1004-8, 2015. [PUBMED Abstract]
- Hancock BJ, Di Lorenzo M, Youssef S, et al.: Childhood primary pulmonary neoplasms. J Pediatr Surg 28 (9): 1133-6, 1993. [PUBMED Abstract]
- Vadasz P, Palffy G, Egervary M, et al.: Diagnosis and treatment of bronchial carcinoid tumors: clinical and pathological review of 120 operated patients. Eur J Cardiothorac Surg 7 (1): 8-11, 1993. [PUBMED Abstract]
- Kulke MH, Mayer RJ: Carcinoid tumors. N Engl J Med 340 (11): 858-68, 1999. [PUBMED Abstract]
- Oliaro A, Filosso PL, Donati G, et al.: Atypical bronchial carcinoids. Review of 46 patients. J Cardiovasc Surg (Torino) 41 (1): 131-5, 2000. [PUBMED Abstract]
- Moraes TJ, Langer JC, Forte V, et al.: Pediatric pulmonary carcinoid: a case report and review of the literature. Pediatr Pulmonol 35 (4): 318-22, 2003. [PUBMED Abstract]
- Al-Qahtani AR, Di Lorenzo M, Yazbeck S: Endobronchial tumors in children: Institutional experience and literature review. J Pediatr Surg 38 (5): 733-6, 2003. [PUBMED Abstract]
- Roby BB, Drehner D, Sidman JD: Pediatric tracheal and endobronchial tumors: an institutional experience. Arch Otolaryngol Head Neck Surg 137 (9): 925-9, 2011. [PUBMED Abstract]
- Varela P, Pio L, Torre M: Primary tracheobronchial tumors in children. Semin Pediatr Surg 25 (3): 150-5, 2016. [PUBMED Abstract]
- Potter SL, HaDuong J, Okcu F, et al.: Pediatric Bronchial Carcinoid Tumors: A Case Series and Review of the Literature. J Pediatr Hematol Oncol 41 (1): 67-70, 2019. [PUBMED Abstract]
Clinical Presentation
When epithelial cancers of the lung occur, they tend to be of advanced stage. Prognosis is dependent on both histology and stage.[1]
The presenting symptoms of tracheobronchial tumors are usually caused by an incomplete tracheobronchial obstruction and include the following:
- Cough.
- Recurrent pneumonitis.
- Hemoptysis.
Because of difficulties in diagnosis, symptoms are frequently present for months. Occasionally, children with wheezing are treated for asthma, and diagnosis can be delayed for as long as 4 to 5 years.[2]
Metastatic lesions are reported in approximately 6% of patients with carcinoid tumors, and recurrences are reported in 2% of cases. Atypical carcinoid tumors are rare but more aggressive, and 50% of these patients present with metastatic disease at diagnosis.[1,3] One study reported a single child with a carcinoid tumor and metastatic disease who developed the classic carcinoid syndrome.[4] Octreotide nuclear scans may demonstrate uptake of radioactivity by the tumor or lymph nodes, suggesting metastatic spread.
References
- Lal DR, Clark I, Shalkow J, et al.: Primary epithelial lung malignancies in the pediatric population. Pediatr Blood Cancer 45 (5): 683-6, 2005. [PUBMED Abstract]
- Abuzetun JY, Hazin R, Suker M, et al.: Primary squamous cell carcinoma of the lung with bony metastasis in a 13-year-old boy: case report and review of literature. J Pediatr Hematol Oncol 30 (8): 635-7, 2008. [PUBMED Abstract]
- Rizzardi G, Marulli G, Calabrese F, et al.: Bronchial carcinoid tumours in children: surgical treatment and outcome in a single institution. Eur J Pediatr Surg 19 (4): 228-31, 2009. [PUBMED Abstract]
- Lack EE, Harris GB, Eraklis AJ, et al.: Primary bronchial tumors in childhood. A clinicopathologic study of six cases. Cancer 51 (3): 492-7, 1983. [PUBMED Abstract]
Diagnostic Evaluation
The management of tracheobronchial tumors is somewhat controversial because tracheobronchial tumors are usually visible endoscopically.
Biopsy of these lesions may be hazardous because of the risk of hemorrhage. New endoscopic techniques have allowed biopsy to be performed more safely.[1,2] However, endoscopic resection is not recommended except in highly selected cases.[2-4]
Bronchography or computed tomography may be helpful to determine the degree of bronchiectasis distal to the obstruction since the degree of pulmonary destruction may influence surgical therapy.[5]
References
- Roby BB, Drehner D, Sidman JD: Pediatric tracheal and endobronchial tumors: an institutional experience. Arch Otolaryngol Head Neck Surg 137 (9): 925-9, 2011. [PUBMED Abstract]
- Malkan AD, Sandoval JA: Controversial tumors in pediatric surgical oncology. Curr Probl Surg 51 (12): 478-520, 2014. [PUBMED Abstract]
- Luckraz H, Amer K, Thomas L, et al.: Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg 132 (1): 113-5, 2006. [PUBMED Abstract]
- Varela P, Pio L, Torre M: Primary tracheobronchial tumors in children. Semin Pediatr Surg 25 (3): 150-5, 2016. [PUBMED Abstract]
- Ahel V, Zubovic I, Rozmanic V: Bronchial adenoid cystic carcinoma with saccular bronchiectasis as a cause of recurrent pneumonia in children. Pediatr Pulmonol 12 (4): 260-2, 1992. [PUBMED Abstract]
Prognosis
With the exception of rhabdomyosarcomas, tracheobronchial tumors of all histological types are associated with an excellent prognosis after surgical resection in children, even in the presence of local invasion.[1,2]; [3][Level of evidence B4]
References
- Soga J, Yakuwa Y: Bronchopulmonary carcinoids: An analysis of 1,875 reported cases with special reference to a comparison between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg 5 (4): 211-9, 1999. [PUBMED Abstract]
- Fauroux B, Aynie V, Larroquet M, et al.: Carcinoid and mucoepidermoid bronchial tumours in children. Eur J Pediatr 164 (12): 748-52, 2005. [PUBMED Abstract]
- Redlich A, Wechsung K, Boxberger N, et al.: Extra-appendiceal neuroendocrine neoplasms in children - data from the GPOH-MET 97 Study. Klin Padiatr 225 (6): 315-9, 2013. [PUBMED Abstract]
Treatment of Childhood Tracheobronchial Tumors
The treatment of choice is conservative pulmonary resection, including sleeve segmental resection (when feasible), with the removal of the involved lymphatics.[1,2]; [3][Level of evidence B4]
Chemotherapy and radiation therapy are not indicated for tracheobronchial tumors, unless evidence of metastasis is documented or the tumor is a rhabdomyosarcoma.
Treatment options for tracheobronchial tumors, according to histological type, are as follows:
Carcinoid Tumors (Neuroendocrine Tumors of the Bronchus)
The treatment of choice is surgical resection with lymph node sampling. The overall survival rate is higher than 90% for these patients.[4-6] One study reported no deaths among patients who did not have lymph node involvement, and the 10-year survival rate was 89% for patients with one or more involved lymph nodes.[6]
Mucoepidermoid Carcinomas
The recommended treatment is open surgical resection and lymph node sampling. Endoscopic resection is not recommended.[7,8]
Inflammatory Myofibroblastic Tumors
The treatment of choice is surgery. However, if the tumor has an ALK variant, treatment with crizotinib may be effective.[8-11]
Rhabdomyosarcomas
Surgery is not indicated. These tumors are very responsive to chemotherapy and radiation therapy, even with lymph node metastasis.[8] For more information, see Childhood Rhabdomyosarcoma Treatment.
Granular Cell Tumors
For information about other neuroendocrine carcinoid tumors, see Pediatric Gastrointestinal Neuroendocrine Tumors Treatment.
References
- Gaissert HA, Mathisen DJ, Grillo HC, et al.: Tracheobronchial sleeve resection in children and adolescents. J Pediatr Surg 29 (2): 192-7; discussion 197-8, 1994. [PUBMED Abstract]
- Jalal A, Jeyasingham K: Bronchoplasty for malignant and benign conditions: a retrospective study of 44 cases. Eur J Cardiothorac Surg 17 (4): 370-6, 2000. [PUBMED Abstract]
- Redlich A, Wechsung K, Boxberger N, et al.: Extra-appendiceal neuroendocrine neoplasms in children - data from the GPOH-MET 97 Study. Klin Padiatr 225 (6): 315-9, 2013. [PUBMED Abstract]
- Travis WD, Rush W, Flieder DB, et al.: Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol 22 (8): 934-44, 1998. [PUBMED Abstract]
- McMullan DM, Wood DE: Pulmonary carcinoid tumors. Semin Thorac Cardiovasc Surg 15 (3): 289-300, 2003. [PUBMED Abstract]
- Raikot SR, Day CN, Boesch RP, et al.: Factors Associated With Long-term Survival in Children With Bronchial and Lung Carcinoid Tumors. J Pediatr Surg 59 (8): 1626-1630, 2024. [PUBMED Abstract]
- Qian X, Sun Z, Pan W, et al.: Childhood bronchial mucoepidermoid tumors: A case report and literature review. Oncol Lett 6 (5): 1409-1412, 2013. [PUBMED Abstract]
- Varela P, Pio L, Torre M: Primary tracheobronchial tumors in children. Semin Pediatr Surg 25 (3): 150-5, 2016. [PUBMED Abstract]
- Jindal A, Bal A, Agarwal R: Inflammatory myofibroblastic tumor of the trachea in the pediatric age group: case report and systematic review of the literature. J Bronchology Interv Pulmonol 22 (1): 58-65, 2015. [PUBMED Abstract]
- Butrynski JE, D'Adamo DR, Hornick JL, et al.: Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363 (18): 1727-33, 2010. [PUBMED Abstract]
- Chavez C, Hoffman MA: Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: A case report and review of the literature. Oncol Lett 5 (5): 1672-1676, 2013. [PUBMED Abstract]
- Finck C, Moront M, Newton C, et al.: Pediatric granular cell tumor of the tracheobronchial tree. J Pediatr Surg 43 (3): 568-70, 2008. [PUBMED Abstract]
- Pernas FG, Younis RT, Lehman DA, et al.: Management of pediatric airway granular cell tumor: role of laryngotracheal reconstruction. Int J Pediatr Otorhinolaryngol 70 (6): 957-63, 2006. [PUBMED Abstract]
Treatment Options Under Clinical Evaluation for Childhood Tracheobronchial Tumors
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Special Considerations for the Treatment of Children With Cancer
Cancer in children and adolescents is rare, although the overall incidence has slowly increased since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following pediatric specialists and others to ensure that children receive treatment, supportive care, and rehabilitation to achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgeons.
- Pathologists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists and hematologists.
- Ophthalmologists.
- Rehabilitation specialists.
- Pediatric oncology nurses.
- Social workers.
- Child-life professionals.
- Psychologists.
- Nutritionists.
For specific information about supportive care for children and adolescents with cancer, see the summaries on Supportive and Palliative Care.
The American Academy of Pediatrics has outlined guidelines for pediatric cancer centers and their role in the treatment of children and adolescents with cancer.[2] At these centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate is offered to most patients and their families. Clinical trials for children and adolescents diagnosed with cancer are generally designed to compare potentially better therapy with current standard therapy. Other types of clinical trials test novel therapies when there is no standard therapy for a cancer diagnosis. Most of the progress in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2020, childhood cancer mortality decreased by more than 50%.[3-5] Childhood and adolescent cancer survivors require close monitoring because side effects of cancer therapy may persist or develop months or years after treatment. For information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors, see Late Effects of Treatment for Childhood Cancer.
Childhood cancer is a rare disease, with about 15,000 cases diagnosed annually in the United States in individuals younger than 20 years.[6] The U.S. Rare Diseases Act of 2002 defines a rare disease as one that affects populations smaller than 200,000 people in the United States. Therefore, all pediatric cancers are considered rare.
The designation of a rare tumor is not uniform among pediatric and adult groups. In adults, rare cancers are defined as those with an annual incidence of fewer than six cases per 100,000 people. They account for up to 24% of all cancers diagnosed in the European Union and about 20% of all cancers diagnosed in the United States.[7,8] In children and adolescents, the designation of a rare tumor is not uniform among international groups, as follows:
- A consensus effort between the European Union Joint Action on Rare Cancers and the European Cooperative Study Group for Rare Pediatric Cancers estimated that 11% of all cancers in patients younger than 20 years could be categorized as very rare. This consensus group defined very rare cancers as those with annual incidences of fewer than two cases per 1 million people. However, three additional histologies (thyroid carcinoma, melanoma, and testicular cancer) with incidences of more than two cases per 1 million people were also included in the very rare group due to a lack of knowledge and expertise in the management of these tumors.[9]
- The Children's Oncology Group defines rare pediatric cancers as those listed in the International Classification of Childhood Cancer subgroup XI, which includes thyroid cancers, melanomas and nonmelanoma skin cancers, and multiple types of carcinomas (e.g., adrenocortical carcinomas, nasopharyngeal carcinomas, and most adult-type carcinomas such as breast cancers and colorectal cancers).[10] These diagnoses account for about 5% of the cancers diagnosed in children aged 0 to 14 years and about 27% of the cancers diagnosed in adolescents aged 15 to 19 years.[4]
Most cancers in subgroup XI are either melanomas or thyroid cancers, with other cancer types accounting for only 2% of the cancers diagnosed in children aged 0 to 14 years and 9.3% of the cancers diagnosed in adolescents aged 15 to 19 years.
These rare cancers are extremely challenging to study because of the relatively few patients with any individual diagnosis, the predominance of rare cancers in the adolescent population, and the small number of clinical trials for adolescents with rare cancers.
References
- Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010. [PUBMED Abstract]
- American Academy of Pediatrics: Standards for pediatric cancer centers. Pediatrics 134 (2): 410-4, 2014. Also available online. Last accessed February 25, 2025.
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed February 25, 2025.
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed December 30, 2024.
- Ward E, DeSantis C, Robbins A, et al.: Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 64 (2): 83-103, 2014 Mar-Apr. [PUBMED Abstract]
- Gatta G, Capocaccia R, Botta L, et al.: Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol 18 (8): 1022-1039, 2017. [PUBMED Abstract]
- DeSantis CE, Kramer JL, Jemal A: The burden of rare cancers in the United States. CA Cancer J Clin 67 (4): 261-272, 2017. [PUBMED Abstract]
- Ferrari A, Brecht IB, Gatta G, et al.: Defining and listing very rare cancers of paediatric age: consensus of the Joint Action on Rare Cancers in cooperation with the European Cooperative Study Group for Pediatric Rare Tumors. Eur J Cancer 110: 120-126, 2019. [PUBMED Abstract]
- Pappo AS, Krailo M, Chen Z, et al.: Infrequent tumor initiative of the Children's Oncology Group: initial lessons learned and their impact on future plans. J Clin Oncol 28 (33): 5011-6, 2010. [PUBMED Abstract]
Latest Updates to This Summary (01/06/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood tracheobronchial tumors. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Tracheobronchial Tumors Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Karen J. Marcus, MD, FACR (Dana-Farber of Boston Children's Cancer Center and Blood Disorders Harvard Medical School)
- William H. Meyer, MD
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Arthur Kim Ritchey, MD (Children's Hospital of Pittsburgh of UPMC)
- Carlos Rodriguez-Galindo, MD (St. Jude Children's Research Hospital)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Tracheobronchial Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lung/hp/child-tracheobronchial-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 31593395]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.