Key Points
- Non-Hodgkin lymphoma (NHL) is the most common form of blood cancer in adults. In 2012, an estimated 70,130 new cases of NHL were diagnosed in the United States.
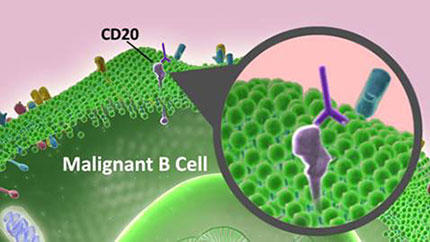
- NHL most often arises from B cells, which are immune cells responsible for producing antibodies.
- The National Cancer Institute (NCI) funded the development of rituximab, one of the first monoclonal antibody (antibodies that target one specific protein) cancer treatments. It plays a key role in increasing survival for patients with NHL.
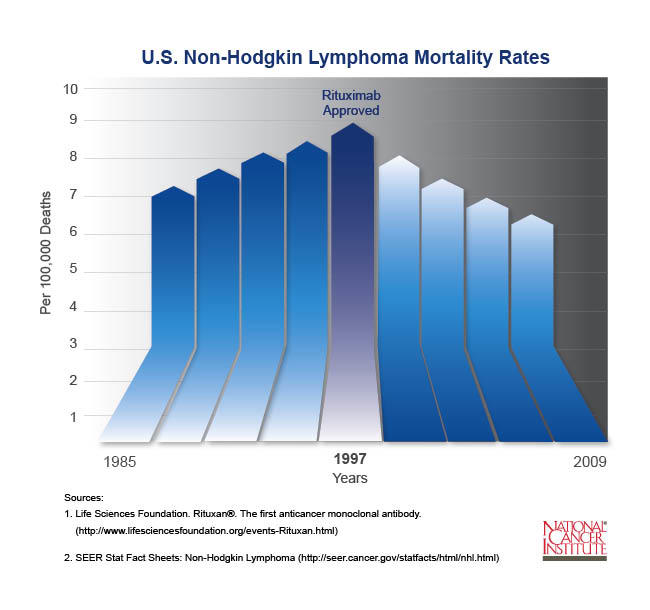
- Prior to the introduction of rituximab in 1997, the number of new cases of NHL increased each year. Since then, deaths from NHL have gone down each year and continue to drop.
Pathway to Discovery
Antibodies are produced by the immune system to help fight infections. Scientists long thought that if antibodies could be created to recognize specific proteins on cancer cells, the immune system could be used to destroy the cancer. Researchers also thought that by targeting cancer cells and avoiding destructive effects on normal cells, treatment might be more effective and with fewer harmful side effects than traditional chemotherapy.
In 1975, NCI-funded researchers César Milstein, Ph.D., and Georges Köhler, Ph.D., took the first steps toward creating such a treatment by producing antibodies that target one specific portion, or epitope, of a particular protein. These are called monoclonal antibodies. The first test of this treatment occurred in 1980 in a patient with NHL, the most common form of blood cancer in adults. While this first trial did not cure the patient, it paved the way for the use of monoclonal antibodies as a cancer treatment. Based on this groundbreaking work, Drs. Milstein and Köhler received the Nobel Prize for Physiology or Medicine in 1984.
Later research showed that these antibodies use the body’s immune system to destroy cancer cells. But for this treatment to work, researchers first needed to find a specific protein to target on NHL B cells, a group of white blood cells that are a vital part of the immune system.
In 1988, scientists identified a protein known as CD20 on B cells. Although CD20 is found on both healthy and cancerous B cells, it is not found on immature or developing B cells. Therefore, a treatment that targeted this protein would eliminate both cancerous and healthy mature B cells, but most of the immature B cells would remain to replenish the supply of healthy cells following treatment. With CD20 as a potential antitumor target, Biogen Idec, a biotechnology company, developed rituximab (Rituxan) to specifically bind to CD20 and eliminate cancerous B cells.
Enhancing Cancer Care
In initial studies, almost half of the patients with NHL who did not respond to previous chemotherapy responded to rituximab with either full or partial remission of their cancer. Patients also experienced fewer side effects than with traditional chemotherapy.
Subsequent studies showed that a combination of rituximab with traditional chemotherapy could significantly prolong survival in patients with NHL. Currently, this combination is used as a first-line therapy for several types of NHL.
Rituximab’s significant antitumor activity led to the development of more than a dozen new monoclonal antibody cancer treatments. This type of targeted treatment has changed the course of treatment for many types of cancer. Prior to genomic sequencing (a process that determines the DNA sequence of a specific cell or organism), doctors relied heavily on seeing tumor cells at the microscopic level to determine the type of tumor. Yet, even though tumor cells look alike, they can actually be very different and respond differently to treatment. As researchers learn more about the differences between normal and tumor cells at the molecular level, they are developing treatments to reduce how severe a cancer may be or to eliminate it altogether.
The discovery of rituximab paved the way for targeted treatments that are revolutionizing cancer treatment and improving patient outcomes every day. Some important monoclonal antibody treatments that have followed in rituximab’s footsteps include trastuzumab (Herceptin) for breast and gastric cancer, ipilimumab (Yervoy) for melanoma, and bevacizumab (Avastin) for colon cancer.
Turning Discovery into Health
The success of rituximab and other monoclonal antibodies in treating cancer has fostered great interest in expanding the use of antibody treatments.
One area showing promise is called immunoconjugate therapy. This treatment links antibodies to a second drug or radioactive molecule. Scientists think this approach can be used to target cancer cells directly to destroy them. Ideally, this treatment would spare healthy cells and prevent many of the serious side effects often caused by chemotherapy.
The potential for this approach in cancer treatment has already been realized. By linking radioactive iodine-131 to the anti-CD20 monoclonal antibody, GlaxoSmithKlein, a pharmaceutical company, developed tositumomab (Bexxar), which delivers radiation specifically to B cells, thereby limiting cell death to B cells alone. In 2003, the FDA approved the use of this drug in patients with NHL.
Because rituximab targets B cells, researchers want to know if it also can be used to treat other similar B-cell type diseases, including autoimmune diseases.
Rituximab has already been approved for the treatment of rheumatoid arthritis, where it has been shown to greatly reduce symptoms and prevent joint damage. Currently, clinical trials are looking at whether rituximab also can help people with multiple sclerosis and systemic lupus erythematosus.
Research to Practice: NCI's Role
Prior to the introduction of rituximab in 1997, the incidence of NHL was steadily increasing. Since then, mortality rates have declined by about three percent each year and continue to drop.
NCI supported research to determine which patients may benefit most from rituximab treatment and which combinations of chemotherapy work best to improve survival in patients with NHL.
NCI also funded a broad portfolio of research that uncovered how tumor cells work, as well as clinical trials that demonstrated the effectiveness of monoclonal antibodies as a targeted treatment against cancer cells.
Key Takeaway
With the discovery of rituximab, more than 70 percent of patients diagnosed with NHL now live five years past their initial diagnosis. Rituximab paved the way for targeted cancer treatments. These targeted treatments have revolutionized cancer care and improve patient outcomes every day.
Selected Resources
American Cancer Society. Immunotherapy: Monoclonal Antibodies. 2013.
American Cancer Society. Non-Hodgkin Lymphoma. 2013.
Dotan E, Aggarwal C, Smith MR. Impact of rituximab (Rituxan) on the treatment of B-cell non-Hodgkin's lymphoma.PT. 2010;35(3):148-157. [PUBMED Abstract]
Hauptrock B, Hess G. Rituximab in the treatment of non-Hodgkin's lymphoma.Biologics. 2008;2(4):619-633 [PUBMED Central]
Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495-497. http://www.nature.com/nature/journal/v256/n5517/abs/256495a0.html
Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90(6):2188-2195. [PUBMED Abstract]
Nadler LM, Stashenko P, Hardy R, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40: 3147. [PUBMED Abstract]
National Cancer Institute. Cancer Topics: Non-Hodgkin Lymphomas. http://www.cancer.gov/types/lymphoma
National Cancer Institute. Cancer Drug Information: Rituximab. http://www.cancer.gov/about-cancer/treatment/drugs/rituximab
Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clinical Oncology. 2008;26(1):1774-1777. http://jco.ascopubs.org/content/26/11/1774.full
Tedder TF, Streuli M, Schlossman SF, Saito H. Isolation and structure of a cDNA encoding the B1 (CD20) cell-surface antigen of human B lymphocytes. Proc Natl Acad Sci USA. 1988;85(1):208–212. [PUBMED Abstract]