Multiple Endocrine Neoplasia Type 2 (MEN2) (PDQ®)–Health Professional Version
Brief Clinical Description of Multiple Endocrine Neoplasia Type 2 (MEN2)
MEN2 is caused by pathogenic variants in the RET gene. MEN2 is distinct from two similarly named syndromes, Multiple Endocrine Neoplasia Type 1 (MEN1) and Multiple Endocrine Neoplasia Type 4 (MEN4). The endocrine disorders observed in MEN2 are medullary thyroid cancer (MTC); its precursor, C-cell hyperplasia (CCH) (referred to as C-cell neoplasia or C-cell carcinoma in situ in more recent publications)[1]; pheochromocytoma (PHEO); and parathyroid adenomas and/or hyperplasia. MEN2-associated MTC is often bilateral and/or multifocal and arises in the background of CCH. In contrast, sporadic MTC is typically unilateral and/or unifocal. Because approximately 75% to 80% of sporadic cases also have associated CCH, this histopathologic feature cannot be used as a predictor of familial disease.[2] Metastatic spread of MTC to regional lymph nodes (i.e., perithyroidal, paratracheal, jugular chain, and upper mediastinum) or to distant sites, such as the liver, is common in patients who present with a palpable thyroid mass or diarrhea.[3,4] When thyroidectomy is performed before local nodal metastases occur, distant metastases are rare.[5] Metastatic PHEOs have not been reported in individuals with MEN2.[6] In MEN2, parathyroid abnormalities can range from benign parathyroid adenomas or multigland hyperplasia to clinically evident hyperparathyroidism with hypercalcemia and renal stones.
The understanding of MEN2's natural history continues to evolve. Clinical observations suggest that the natural history of MEN2 (particularly the penetrance of MTC) is variable. The manifestations of MEN2 could be subject to modifying effects from specific RET pathogenic variants, other genes, behavioral factors, or environmental exposures.[7] For example, it is widely reported that most patients with MEN2 will develop MTC. A seminal study from 1989 (published before the RET gene was discovered) found that screening at-risk family members for MTC with pentagastrin stimulation increased penetrance of MTC to 93%.[8] In contrast, penetrance ranged from 41% to 65% when it was solely based on MTC clinical presentation.[9] This difference suggests that individuals with MEN2 have MTC with varying natural history, penetrance, and aggressiveness. As research continues, the understanding of genotype-phenotype correlations and recommendations for the optimal age for thyroidectomy continue to change.[10]
MEN2 can be divided into two subtypes: multiple endocrine neoplasia type 2A (MEN2A) and multiple endocrine neoplasia type 2B (MEN2B). Familial medullary thyroid cancer (FMTC) is generally considered to be part of MEN2A, although it was historically thought to be a separate MEN2 subtype.
Current stratification has moved away from a solely phenotype-based classification to one that is based on genotype (i.e., the pathogenic variant) and phenotype.[11] The MEN2A syndrome is further classified on the basis of the presence of associated conditions. For example, classical MEN2A includes those with MTC, PHEO, and/or hyperparathyroidism. Additional categories include MEN2A with cutaneous lichen amyloidosis, MEN2A with Hirschsprung disease, and FMTC (presence of a RET germline pathogenic variant and MTC but no family history of PHEO or hyperparathyroidism).[1] Classifying a patient or family by MEN2 subtype is useful in determining prognosis and management.
The prevalence of MEN2 has been estimated to be approximately 1 in 35,000 individuals.[12] The vast majority of MEN2 cases are MEN2A.
References
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015. [PUBMED Abstract]
- Kaserer K, Scheuba C, Neuhold N, et al.: Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am J Surg Pathol 25 (10): 1245-51, 2001. [PUBMED Abstract]
- Robbins J, Merino MJ, Boice JD, et al.: Thyroid cancer: a lethal endocrine neoplasm. Ann Intern Med 115 (2): 133-47, 1991. [PUBMED Abstract]
- Moley JF, Debenedetti MK, Dilley WG, et al.: Surgical management of patients with persistent or recurrent medullary thyroid cancer. J Intern Med 243 (6): 521-6, 1998. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Exceptionality of Distant Metastasis in Node-Negative Hereditary and Sporadic Medullary Thyroid Cancer: Lessons Learned. J Clin Endocrinol Metab 106 (8): e2968-e2979, 2021. [PUBMED Abstract]
- Thosani S, Ayala-Ramirez M, Palmer L, et al.: The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab 98 (11): E1813-9, 2013. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Lymph node metastasis in hereditary medullary thyroid cancer is independent of the underlying RET germline mutation. Eur J Surg Oncol 47 (4): 920-923, 2021. [PUBMED Abstract]
- Easton DF, Ponder MA, Cummings T, et al.: The clinical and screening age-at-onset distribution for the MEN-2 syndrome. Am J Hum Genet 44 (2): 208-15, 1989. [PUBMED Abstract]
- Ponder BA, Ponder MA, Coffey R, et al.: Risk estimation and screening in families of patients with medullary thyroid carcinoma. Lancet 1 (8582): 397-401, 1988. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Genotype-specific progression of hereditary medullary thyroid cancer. Hum Mutat 39 (6): 860-869, 2018. [PUBMED Abstract]
- Machens A, Lorenz K, Dralle H: Constitutive RET tyrosine kinase activation in hereditary medullary thyroid cancer: clinical opportunities. J Intern Med 266 (1): 114-25, 2009. [PUBMED Abstract]
- DeLellis RA, Lloyd RV, Heitz PU, et al., eds.: Pathology and Genetics of Tumours of Endocrine Organs. IARC Press, 2004. World Health Organization classification of tumours, vol. 8.
Clinical Features of Multiple Endocrine Neoplasia Type 2 (MEN2)
The following endocrine disorders are observed in MEN2:[1]
- Medullary thyroid cancer (MTC).
- C-cell hyperplasia (CCH), MTC's precursor.
- Pheochromocytoma (PHEO).
- Parathyroid adenomas and/or parathyroid hyperplasia.
Medullary Thyroid Cancer (MTC) and C-Cell Hyperplasia (CCH)
MTC accounts for 1% to 2% of new cases of thyroid cancer diagnosed annually in the United States.[2] Approximately 75% of MTC cases diagnosed in the United States are sporadic (i.e., they occur in the absence of a family history of either MTC or other endocrine abnormalities seen in MEN2). The peak incidence of the sporadic form occurs in the fifth and sixth decades of life.[3,4]
MTC originates in calcitonin-producing cells (C-cells) of the thyroid gland. MTC is diagnosed when nests of C-cells extend beyond the basement membrane and infiltrate and destroy thyroid follicles. CCH is a controversial diagnosis, but most pathologists agree that it is defined as more than seven C-cells per cluster, complete follicles surrounded by C-cells, and C-cells in a distribution beyond normal anatomical location.[1,5-7] Individuals with RET pathogenic variants and CCH are at substantially increased risk of progressing to MTC. MTC and CCH are suspected in the presence of an elevated plasma calcitonin concentration. Genetic testing and calcitonin screening are associated with improved MTC survival when compared with MTC identified via symptoms.[8-11]
In the absence of a positive family history, MEN2 may be suspected when MTC occurs at an early age or is bilateral or multifocal. While small series of apparently sporadic MTC cases have suggested a higher prevalence of germline RET pathogenic variants,[12,13] larger series indicate a prevalence range of 1% to 7%.[14,15] Based on these data, testing for pathogenic variants in the RET gene is widely recommended for all cases of MTC.[1,16] For more information, see the Genetic Counseling and Genetic Testing section.
Pheochromocytoma (PHEO)
The risk of developing a PHEO is elevated in individuals with multiple endocrine neoplasia type 2A (MEN2A) and multiple endocrine neoplasia type 2B (MEN2B). However, the degree of risk depends on which specific RET pathogenic variant is involved. For more information about PHEO risks for specific RET pathogenic variants, see Table 1.
PHEOs arise from the catecholamine-producing chromaffin cells of the adrenal medulla. They are relatively rare tumors and are suspected among patients with refractory hypertension or when biochemical screening reveals elevated excretion of catecholamines and catecholamine metabolites (i.e., norepinephrine, epinephrine, metanephrine, and vanillylmandelic acid) in 24-hour urine collections or plasma.[17-22] When biochemical screening in an individual who has or is at risk of MEN2 suggests PHEO, localization studies, such as magnetic resonance imaging (MRI) or computed tomography, can be performed.[23] Confirmation of the diagnosis can be made using iodine I 131-metaiodobenzylguanidine scintigraphy or positron emission tomography imaging.[23-26] For more information about diagnostics and biochemical testing for PHEOs, see the Diagnostics section in Pheochromocytoma and Paraganglioma Treatment.
Primary Hyperparathyroidism (PHPT)
Although PHPT has not been associated with MEN2B, the risk of developing PHPT in MEN2A depends on which specific RET pathogenic variant is involved. For more information about PHPT risks for specific RET pathogenic variants, see Table 1.
Hereditary PHPT is typically multiglandular, presents earlier in life, and can have histologic evidence of both adenoma and glandular hyperplasia. Most patients with MEN2-related parathyroid disease are either asymptomatic or are diagnosed incidentally during preoperative planning or thyroidectomy. Typically, hypercalcemia (when present) is mild. However, hypercalcemia may be associated with nephrolithiasis and increased urinary excretion of calcium.[27]
For information about hereditary syndromes associated with PHPT, see the Genetic Testing and Differential Diagnosis for MEN1 section in Genetics of Endocrine and Neuroendocrine Neoplasias.
Clinical Subtypes of Multiple Endocrine Neoplasia Type 2 (MEN2)
Diagnosis of the two MEN2 clinical subtypes relies on a combination of clinical findings, family history, and molecular genetic testing of the RET gene.
MEN2A
Most patients with MEN2 have the MEN2A subtype.
Classical MEN2A
MEN2A is diagnosed clinically by the occurrence of two specific endocrine tumors in addition to MTC: PHEO and/or parathyroid adenoma and/or hyperplasia in a single individual or in close relatives.[1]
The classical MEN2A subtype comprises about 60% to 90% of MEN2 cases. Since genetic testing for RET pathogenic variants has become available, about 95% of individuals with MEN2A screen positive for MTC.[24,28-30]
MTC is generally the first manifestation of MEN2A. In asymptomatic at-risk individuals, stimulation testing may reveal elevated plasma calcitonin levels and the presence of CCH or MTC.[24,29] In families with MEN2A, the biochemical manifestations of MTC generally appear between the ages of 5 years and 25 years (mean, 15 y).[24] If presymptomatic screening is not performed, MTC typically presents as a neck mass or neck pain between the ages of about age 5 years and 20 years. More than 50% of such patients have cervical lymph node metastases.[3] Diarrhea, the most frequent systemic symptom, occurs in patients with a markedly elevated plasma calcitonin level or bulky disease and/or hepatic metastases and implies a poor prognosis.[1,3,31,32] Up to 30% of patients with MTC present with diarrhea and advanced disease.[33]
MEN2-associated PHEOs are more often bilateral, multifocal, and associated with extratumoral medullary hyperplasia.[34-36] They also have an earlier age of onset and are less likely to be malignant than their sporadic counterparts.[34,37] MEN2-associated PHEOs usually present after MTC, typically with intractable hypertension.[38]
Hyperparathyroidism in individuals with MEN2 is typically asymptomatic or associated with only mild elevations in calcium.[33,39] A series of 56 patients with MEN2-related hyperparathyroidism has been reported by the French Calcitonin Tumors Study Group.[39] The median age at diagnosis was 38 years, documenting that this disorder is rarely the first manifestation of MEN2. Parathyroid abnormalities were found concomitantly with surgery for MTC in 43 patients (77%). Two-thirds of the patients were asymptomatic. Among the 53 parathyroid glands removed surgically, there were 24 single adenomas, 4 double adenomas, and 25 hyperplastic glands.
MEN2A with cutaneous lichen amyloidosis
A small number of families with MEN2A have pruritic skin lesions known as cutaneous lichen amyloidosis. This lichenoid skin lesion is located over the upper portion of the back and may appear before the onset of MTC.[40,41] For more information, see Table 1.
MEN2A with Hirschsprung disease (HSCR)
HSCR, a disorder of the enteric plexus of the colon that typically results in enlargement of the bowel and constipation or obstipation in neonates, occurs in a small number of individuals with MEN2A-associated RET pathogenic variants.[42] Pathogenic variants at specific cysteine residues in exon 10 (i.e., codons 609, 618, and 620) are most commonly associated with HSCR, although individuals with pathogenic variants in other exons can still be affected.[43] For more information, see Table 1. HSCR can occur outside of a diagnosis of MEN2A, and infants with HSCR may benefit from their own genetic evaluation, regardless of the likelihood of MEN2A. Up to 40% of familial cases of HSCR and 3% to 7% of sporadic cases are associated with germline pathogenic variants in the RET proto-oncogene.[44,45] Certain loss-of-function RET variants have been associated with isolated HSCR,[46] indicating that not all individuals with HSCR and a germline RET variant necessarily have MEN2A.
Figure 1 depicts some of the classic manifestations of MEN2A in a family.
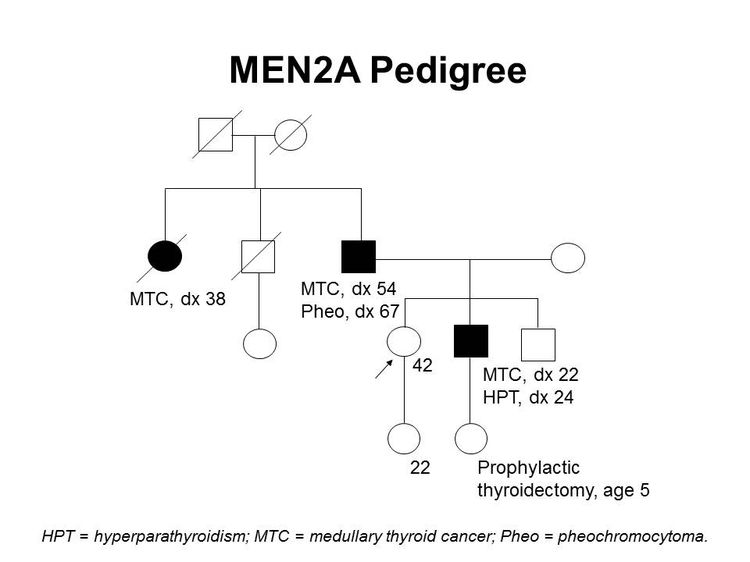
Familial medullary thyroid cancer (FMTC)
Up to 50% of MEN2A cases are of the FMTC subtype, and are defined as families or individuals with germline RET pathogenic variants and MTC alone in the absence of PHEO or parathyroid adenoma/hyperplasia.[1] This definition replaces previous classification of FMTC as a stand-alone diagnosis.[1] Previously, misclassification of families as having FMTC (because of too-small family size or later onset of other manifestations of MEN2A) could result in overlooking the risk of PHEO, a disease with significant morbidity and mortality. For this reason, FMTC is now considered a subtype of MEN2A in which there is a lack of or delay in the onset of the other (nonthyroidal) manifestations of the MEN2A syndrome.[47] Current management guidelines [1] recommend that patients thought to have pure FMTC also be screened for PHEO and hyperparathyroidism.
MEN2B
The MEN2B subtype comprises about 5% of MEN2 cases. MEN2B is characterized by the development of aggressive MTC at a young age (in all patients), the presence of mucosal neuromas, gastrointestinal ganglioneuromatosis, medullated corneal nerve fibers, and distinct physical features.[48-51] PHEOs occur in about 50% of MEN2B cases. About half of these cases have multiple PHEOs, which are often bilateral. Clinically apparent parathyroid disease is very uncommon in individuals with MEN2B.[28,48]
In cases of de novo pathogenic variants, the diagnosis of MEN2B is often delayed, after the development of MTC. The MTC is often fatal, particularly in the presence of metastatic disease, which is common at the time of diagnosis. It is important for pediatricians to recognize the endocrine and nonendocrine clinical manifestations of the syndrome as an earlier diagnosis may result in lifesaving treatment of MTC, before metastatic spread.[52]
Patients with MEN2B who do not undergo thyroidectomy at approximately age 1 year are likely to develop metastatic MTC at an early age. Before intervention with early risk-reducing thyroidectomy, the average age at death in patients with MEN2B was 21 years.
Patients with MEN2B may be identified in infancy or early childhood by a distinctive facial appearance and the presence of mucosal neuromas on the anterior dorsal surface of the tongue, palate, or pharynx.[28,48] The lips become prominent over time, and submucosal nodules may be present on the vermilion border of the lips. Neuromas of the eyelids may cause thickening and eversion of the upper eyelid margins. Prominent thickened corneal nerves may be seen by slit lamp examination.
Patients with MEN2B may have diffuse ganglioneuromatosis of the gastrointestinal tract with associated symptoms that include abdominal distension, megacolon, constipation, and diarrhea.[53,54] A review of the literature reported constipation as a common symptom in 72.7% of patients with MEN2B. Additionally, gastrointestinal symptoms occurred during the first year of life in 52.3% of patients with MEN2B. Intestinal biopsy led to the diagnosis of ganglioneuromatosis in 27.3% of patients.[55]
About 75% of patients with MEN2B have tall, thin body types, with arms and legs that are proportionately long when compared with their torso/overall height. Patients also present with kyphoscoliosis/lordosis, joint laxity, and decreased subcutaneous fat. Proximal muscle wasting and weakness can also be seen.[50,51]
A retrospective review of the clinical presentation of 35 cases of MEN2B with de novo pathogenic variants treated at a single institution found that 22 cases were diagnosed because of endocrine manifestations of the syndrome.[52] The diagnosis of PHEO, a neck mass, and/or skeletal abnormalities led to the identification of MTC. The remaining 13 patients presented with a nonendocrine manifestation, including oral neuromas, corneal nerve abnormalities, persistent diarrhea, failure to thrive, or skeletal abnormalities with frequent falls. Of the entire cohort, 21 patients had one or more physician referrals for the evaluation of an MEN2B-related feature, an average of 5 years before the diagnosis of MEN2B.
References
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015. [PUBMED Abstract]
- Howlader N, Noone AM, Krapcho M, et al.: SEER Cancer Statistics Review (CSR) 1975-2017. Bethesda, Md: National Cancer Institute, 2020. Available online. Last accessed February 7, 2025.
- Robbins J, Merino MJ, Boice JD, et al.: Thyroid cancer: a lethal endocrine neoplasm. Ann Intern Med 115 (2): 133-47, 1991. [PUBMED Abstract]
- Gharib H, McConahey WM, Tiegs RD, et al.: Medullary thyroid carcinoma: clinicopathologic features and long-term follow-up of 65 patients treated during 1946 through 1970. Mayo Clin Proc 67 (10): 934-40, 1992. [PUBMED Abstract]
- Guyétant S, Rousselet MC, Durigon M, et al.: Sex-related C cell hyperplasia in the normal human thyroid: a quantitative autopsy study. J Clin Endocrinol Metab 82 (1): 42-7, 1997. [PUBMED Abstract]
- LiVolsi VA: C cell hyperplasia/neoplasia. J Clin Endocrinol Metab 82 (1): 39-41, 1997. [PUBMED Abstract]
- Mete O, Asa SL: Precursor lesions of endocrine system neoplasms. Pathology 45 (3): 316-30, 2013. [PUBMED Abstract]
- Roman S, Lin R, Sosa JA: Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107 (9): 2134-42, 2006. [PUBMED Abstract]
- Modigliani E, Vasen HM, Raue K, et al.: Pheochromocytoma in multiple endocrine neoplasia type 2: European study. The Euromen Study Group. J Intern Med 238 (4): 363-7, 1995. [PUBMED Abstract]
- Bergholm U, Bergström R, Ekbom A: Long-term follow-up of patients with medullary carcinoma of the thyroid. Cancer 79 (1): 132-8, 1997. [PUBMED Abstract]
- Kebebew E, Ituarte PH, Siperstein AE, et al.: Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88 (5): 1139-48, 2000. [PUBMED Abstract]
- Decker RA, Peacock ML, Borst MJ, et al.: Progress in genetic screening of multiple endocrine neoplasia type 2A: is calcitonin testing obsolete? Surgery 118 (2): 257-63; discussion 263-4, 1995. [PUBMED Abstract]
- Kitamura Y, Goodfellow PJ, Shimizu K, et al.: Novel germline RET proto-oncogene mutations associated with medullary thyroid carcinoma (MTC): mutation analysis in Japanese patients with MTC. Oncogene 14 (25): 3103-6, 1997. [PUBMED Abstract]
- Eng C, Mulligan LM, Smith DP, et al.: Low frequency of germline mutations in the RET proto-oncogene in patients with apparently sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 43 (1): 123-7, 1995. [PUBMED Abstract]
- Wohllk N, Cote GJ, Bugalho MM, et al.: Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab 81 (10): 3740-5, 1996. [PUBMED Abstract]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma. Version 2.2023. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free subscription Last accessed December 9, 2024.
- Lenders JW, Pacak K, Walther MM, et al.: Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287 (11): 1427-34, 2002. [PUBMED Abstract]
- Gerlo EA, Sevens C: Urinary and plasma catecholamines and urinary catecholamine metabolites in pheochromocytoma: diagnostic value in 19 cases. Clin Chem 40 (2): 250-6, 1994. [PUBMED Abstract]
- Guller U, Turek J, Eubanks S, et al.: Detecting pheochromocytoma: defining the most sensitive test. Ann Surg 243 (1): 102-7, 2006. [PUBMED Abstract]
- Raber W, Raffesberg W, Bischof M, et al.: Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med 160 (19): 2957-63, 2000. [PUBMED Abstract]
- Sawka AM, Jaeschke R, Singh RJ, et al.: A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88 (2): 553-8, 2003. [PUBMED Abstract]
- Unger N, Pitt C, Schmidt IL, et al.: Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol 154 (3): 409-17, 2006. [PUBMED Abstract]
- Pacak K, Eisenhofer G, Ahlman H, et al.: Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab 3 (2): 92-102, 2007. [PUBMED Abstract]
- Lips CJ, Landsvater RM, Höppener JW, et al.: Clinical screening as compared with DNA analysis in families with multiple endocrine neoplasia type 2A. N Engl J Med 331 (13): 828-35, 1994. [PUBMED Abstract]
- van der Harst E, de Herder WW, Bruining HA, et al.: [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab 86 (2): 685-93, 2001. [PUBMED Abstract]
- Pacak K, Linehan WM, Eisenhofer G, et al.: Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med 134 (4): 315-29, 2001. [PUBMED Abstract]
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PUBMED Abstract]
- Eng C, Clayton D, Schuffenecker I, et al.: The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 276 (19): 1575-9, 1996. [PUBMED Abstract]
- Sanso GE, Domene HM, Garcia R, et al.: Very early detection of RET proto-oncogene mutation is crucial for preventive thyroidectomy in multiple endocrine neoplasia type 2 children: presence of C-cell malignant disease in asymptomatic carriers. Cancer 94 (2): 323-30, 2002. [PUBMED Abstract]
- Yip L, Cote GJ, Shapiro SE, et al.: Multiple endocrine neoplasia type 2: evaluation of the genotype-phenotype relationship. Arch Surg 138 (4): 409-16; discussion 416, 2003. [PUBMED Abstract]
- Rambaud JC, Jian R, Flourié B, et al.: Pathophysiological study of diarrhoea in a patient with medullary thyroid carcinoma. Evidence against a secretory mechanism and for the role of shortened colonic transit time. Gut 29 (4): 537-43, 1988. [PUBMED Abstract]
- Cox TM, Fagan EA, Hillyard CJ, et al.: Rôle of calcitonin in diarrhoea associated with medullary carcinoma of the thyroid. Gut 20 (7): 629-33, 1979. [PUBMED Abstract]
- Raue F, Frank-Raue K, Grauer A: Multiple endocrine neoplasia type 2. Clinical features and screening. Endocrinol Metab Clin North Am 23 (1): 137-56, 1994. [PUBMED Abstract]
- Perren A, Komminoth P: Familial pheochromocytomas and paragangliomas: stories from the sign-out room. Endocr Pathol 17 (4): 337-44, 2006. [PUBMED Abstract]
- Webb TA, Sheps SG, Carney JA: Differences between sporadic pheochromocytoma and pheochromocytoma in multiple endocrime neoplasia, type 2. Am J Surg Pathol 4 (2): 121-6, 1980. [PUBMED Abstract]
- Lips KJ, Van der Sluys Veer J, Struyvenberg A, et al.: Bilateral occurrence of pheochromocytoma in patients with the multiple endocrine neoplasia syndrome type 2A (Sipple's syndrome). Am J Med 70 (5): 1051-60, 1981. [PUBMED Abstract]
- Neumann HP, Berger DP, Sigmund G, et al.: Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med 329 (21): 1531-8, 1993. [PUBMED Abstract]
- Conte-Devolx B, Schuffenecker I, Niccoli P, et al.: Multiple endocrine neoplasia type 2: management of patients and subjects at risk. French Study Group on Calcitonin-Secreting Tumors (GETC). Horm Res 47 (4-6): 221-6, 1997. [PUBMED Abstract]
- Kraimps JL, Denizot A, Carnaille B, et al.: Primary hyperparathyroidism in multiple endocrine neoplasia type IIa: retrospective French multicentric study. Groupe d'Etude des Tumeurs á Calcitonine (GETC, French Calcitonin Tumors Study Group), French Association of Endocrine Surgeons. World J Surg 20 (7): 808-12; discussion 812-3, 1996. [PUBMED Abstract]
- Bugalho MJ, Limbert E, Sobrinho LG, et al.: A kindred with multiple endocrine neoplasia type 2A associated with pruritic skin lesions. Cancer 70 (11): 2664-7, 1992. [PUBMED Abstract]
- Robinson MF, Furst EJ, Nunziata V, et al.: Characterization of the clinical features of five families with hereditary primary cutaneous lichen amyloidosis and multiple endocrine neoplasia type 2. Henry Ford Hosp Med J 40 (3-4): 249-52, 1992. [PUBMED Abstract]
- Romeo G, Ceccherini I, Celli J, et al.: Association of multiple endocrine neoplasia type 2 and Hirschsprung disease. J Intern Med 243 (6): 515-20, 1998. [PUBMED Abstract]
- Mulligan LM, Eng C, Attié T, et al.: Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum Mol Genet 3 (12): 2163-7, 1994. [PUBMED Abstract]
- Decker RA, Peacock ML, Watson P: Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet 7 (1): 129-34, 1998. [PUBMED Abstract]
- Carrasquillo MM, McCallion AS, Puffenberger EG, et al.: Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat Genet 32 (2): 237-44, 2002. [PUBMED Abstract]
- Fewtrell MS, Tam PK, Thomson AH, et al.: Hirschsprung's disease associated with a deletion of chromosome 10 (q11.2q21.2): a further link with the neurocristopathies? J Med Genet 31 (4): 325-7, 1994. [PUBMED Abstract]
- Pacini F, Castagna MG, Cipri C, et al.: Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 22 (6): 475-85, 2010. [PUBMED Abstract]
- Brauckhoff M, Machens A, Hess S, et al.: Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: An exploratory analysis. Surgery 144 (6): 1044-50; discussion 1050-3, 2008. [PUBMED Abstract]
- Castinetti F, Moley J, Mulligan L, et al.: A comprehensive review on MEN2B. Endocr Relat Cancer 25 (2): T29-T39, 2018. [PUBMED Abstract]
- Gorlin RJ, Sedano HO, Vickers RA, et al.: Multiple mucosal neuromas, pheochromocytoma and medullary carcinoma of the thyroid--a syndrome. Cancer 22 (2): 293-9 passim, 1968. [PUBMED Abstract]
- Gorlin RJ, Vickers RA: Multiple mucosal neuromas, pheochromocytoma, medullary carcinoma of the thyroid and marfanoid body build with muscle wasting: reexamination of a syndrome of neural crest malmigration. Birth Defects Orig Artic Ser 7 (6): 69-72, 1971. [PUBMED Abstract]
- Makri A, Akshintala S, Derse-Anthony C, et al.: Multiple Endocrine Neoplasia Type 2B Presents Early in Childhood but Often Is Undiagnosed for Years. J Pediatr 203: 447-449, 2018. [PUBMED Abstract]
- Wells SA, Pacini F, Robinson BG, et al.: Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab 98 (8): 3149-64, 2013. [PUBMED Abstract]
- van den Broek MFM, Rijks EBG, Nikkels PGJ, et al.: Timely diagnosis of multiple endocrine neoplasia 2B by identification of intestinal ganglioneuromatosis: a case series. Endocrine 72 (3): 905-914, 2021. [PUBMED Abstract]
- Gfroerer S, Theilen TM, Fiegel H, et al.: Identification of intestinal ganglioneuromatosis leads to early diagnosis of MEN2B: role of rectal biopsy. J Pediatr Surg 52 (7): 1161-1165, 2017. [PUBMED Abstract]
Diagnosing Multiple Endocrine Neoplasia Type 2 (MEN2)
MEN2 is a well-defined hereditary cancer syndrome. Genetic testing is an important management tool that defines who has an MEN2 diagnosis. It can also provide family members with predictive genetic testing options. There are also rare cases of suspected MEN2 in which a genetic variant has not been identified.
Genetic Counseling and Genetic Testing
MEN2 syndrome is the result of an inherited pathogenic variant in the RET gene, located on chromosome region 10q11.2.[1-3] The RET gene is a proto-oncogene composed of 21 exons over 55 kilobase of genomic material.[4,5] Germline DNA testing for RET pathogenic variants is recommended for all individuals with medullary thyroid cancer (MTC) by the American Thyroid Association and the National Comprehensive Cancer Network.[6-8] Testing is recommended regardless of whether there is a personal or family history suggestive of MEN2. Additionally, MEN2 meets the criteria related to indications for genetic testing for cancer susceptibility outlined by the American Society of Clinical Oncology.[9]
It is critical for pediatricians and other providers who care for infants/children (e.g., gastroenterologists, pathologists, oral health care professionals) to maintain a high index of suspicion when evaluating patients with any clinical manifestations associated with multiple endocrine neoplasia type 2B (MEN2B). In a child or infant, the presence of oral and/or ocular neuromas, gastrointestinal manifestations like severe constipation and/or the need for a rectal biopsy, and/or a tall, lanky body type may warrant further investigation. The identification of these features can prompt early diagnosis of MEN2B and provide the opportunity to prevent or cure MTC.[10,11] Genetic counseling has been recommended for individuals with MTC and any of the following features:[10,12,13]
- Benign oral and submucosal neuromas.
- Elongated face and large lips.
- Ganglioneuromatosis.
- Inability to cry tears (biological mechanism unknown).
While most MTC cases are sporadic, approximately 20% to 25% are hereditary.[14] These hereditary cases are caused by pathogenic variants in the RET proto-oncogene. Between 1% and 10% of individuals with apparently sporadic MTC carry a germline RET pathogenic variant, underscoring the importance of genetic testing for all individuals diagnosed with MTC.[15-17]
There is considerable diversity in the techniques used and the approach to RET pathogenic variant testing among the various laboratories that perform this procedure. Methods used to detect variants in RET include polymerase chain reaction (PCR) followed by restriction enzyme digestion of PCR products, heteroduplex analysis, single-stranded conformation polymorphism analysis, denaturing high-performance liquid chromatography, and DNA sequencing.[18,19] At a minimum, most testing laboratories offer testing using a targeted exon approach; that is, the laboratories look for variants in the exons that are most commonly found to carry variants (exons 10, 11, 13, 14, 15 and 16). Other laboratories offer testing of all RET exons. Differences in variant detection methods are important to consider when selecting a genetic testing laboratory and interpreting the test results. For example, if MEN2 targeted-exon testing is negative in a family that is suspected to have MEN2, sequencing of the remaining exons can be considered. For more information on clinical validity, see Cancer Genetics Risk Assessment and Counseling.
Familial risk assessment
At-risk individuals are defined as first-degree relatives (i.e., parents, siblings, and children) of a person known to have MEN2. Genetic testing can identify people with asymptomatic MEN2. These individuals can consider biochemical screening and early thyroidectomy as preventive measures. All MEN2 subtypes are inherited in an autosomal dominant manner. The risk of inheriting the RET pathogenic variant is 50% in children of individuals with MEN2. Because early detection of at-risk individuals affects medical management, testing children without MEN2 symptoms can be beneficial.[20,21]
Some individuals with MEN2 carry a de novo pathogenic variant; that is, they carry a new pathogenic variant that was not present in previous generations of their family and thus do not have an affected parent. The proportion of individuals with MEN2 who have an affected parent varies by subtype:
- Multiple endocrine neoplasia type 2A (MEN2A): About 95% of affected individuals have an affected parent. Parents of an individual with MEN2A can be evaluated for manifestations of the disorder. In MEN2A cases that are not familial (5%), either de novo pathogenic variants or incomplete penetrance is possible.[22]
- Familial medullary thyroid cancer (FMTC): Multiple family members are affected. Therefore, all affected individuals inherited the RET pathogenic variant from a parent.
- Multiple endocrine neoplasia type 2B (MEN2B): About 50% of affected individuals have de novo RET pathogenic variants, and 50% inherited the pathogenic variant from a parent.[23,24] The majority of de novo pathogenic variants occur on the paternally-inherited allele, but de novo pathogenic variants on the maternally-inherited allele have also been reported.[25]
The risk of siblings having MEN2 depends on the genetic status of the parent, which can be clarified by pedigree analysis and/or DNA-based testing. In situations of apparent de novo pathogenic variants, germline mosaicism in an apparently unaffected parent must be considered, even though such an occurrence has not yet been reported.
In rare circumstances, genetic testing is negative in a patient with a personal or family history suggestive of MEN2. Negative pathogenic variant analysis in at-risk relatives is informative only after a disease-causing pathogenic variant has been identified in an affected relative. Familial screening recommendations are personalized, and updated genetic testing is recommended in families suspected of having MEN2 (in which a RET pathogenic variant has not been identified).
For more information about clinical management of at-risk individuals, see the Genotype-Phenotype Correlations and Risk Stratification in MEN2 section.
References
- Gardner E, Papi L, Easton DF, et al.: Genetic linkage studies map the multiple endocrine neoplasia type 2 loci to a small interval on chromosome 10q11.2. Hum Mol Genet 2 (3): 241-6, 1993. [PUBMED Abstract]
- Mole SE, Mulligan LM, Healey CS, et al.: Localisation of the gene for multiple endocrine neoplasia type 2A to a 480 kb region in chromosome band 10q11.2. Hum Mol Genet 2 (3): 247-52, 1993. [PUBMED Abstract]
- Takahashi M, Ritz J, Cooper GM: Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42 (2): 581-8, 1985. [PUBMED Abstract]
- Kwok JB, Gardner E, Warner JP, et al.: Structural analysis of the human ret proto-oncogene using exon trapping. Oncogene 8 (9): 2575-82, 1993. [PUBMED Abstract]
- Myers SM, Eng C, Ponder BA, et al.: Characterization of RET proto-oncogene 3' splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene 11 (10): 2039-45, 1995. [PUBMED Abstract]
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015. [PUBMED Abstract]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma. Version 2.2023. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free subscription Last accessed December 9, 2024.
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PUBMED Abstract]
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33 (31): 3660-7, 2015. [PUBMED Abstract]
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015. [PUBMED Abstract]
- van den Broek MFM, Rijks EBG, Nikkels PGJ, et al.: Timely diagnosis of multiple endocrine neoplasia 2B by identification of intestinal ganglioneuromatosis: a case series. Endocrine 72 (3): 905-914, 2021. [PUBMED Abstract]
- Brauckhoff M, Machens A, Hess S, et al.: Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: An exploratory analysis. Surgery 144 (6): 1044-50; discussion 1050-3, 2008. [PUBMED Abstract]
- Bashford MT, Kohlman W, Everett J, et al.: Addendum: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 21 (12): 2844, 2019. [PUBMED Abstract]
- Romei C, Ciampi R, Elisei R: A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 12 (4): 192-202, 2016. [PUBMED Abstract]
- Romei C, Cosci B, Renzini G, et al.: RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin Endocrinol (Oxf) 74 (2): 241-7, 2011. [PUBMED Abstract]
- Elisei R, Cosci B, Romei C, et al.: Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 93 (3): 682-7, 2008. [PUBMED Abstract]
- Sarika HL, Papathoma A, Garofalaki M, et al.: Genetic screening of patients with medullary thyroid cancer in a referral center in Greece during the past two decades. Eur J Endocrinol 172 (4): 501-9, 2015. [PUBMED Abstract]
- Mathiesen JS, Kroustrup JP, Vestergaard P, et al.: Distribution of RET Mutations in Multiple Endocrine Neoplasia 2 in Denmark 1994-2014: A Nationwide Study. Thyroid 27 (2): 215-223, 2017. [PUBMED Abstract]
- Wells SA: Advances in the management of MEN2: from improved surgical and medical treatment to novel kinase inhibitors. Endocr Relat Cancer 25 (2): T1-T13, 2018. [PUBMED Abstract]
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010. [PUBMED Abstract]
- Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. Am J Hum Genet 57 (5): 1233-41, 1995. [PUBMED Abstract]
- Schuffenecker I, Ginet N, Goldgar D, et al.: Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma. Le Groupe d'Etude des Tumeurs a Calcitonine. Am J Hum Genet 60 (1): 233-7, 1997. [PUBMED Abstract]
- Norum RA, Lafreniere RG, O'Neal LW, et al.: Linkage of the multiple endocrine neoplasia type 2B gene (MEN2B) to chromosome 10 markers linked to MEN2A. Genomics 8 (2): 313-7, 1990. [PUBMED Abstract]
- Carlson KM, Bracamontes J, Jackson CE, et al.: Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am J Hum Genet 55 (6): 1076-82, 1994. [PUBMED Abstract]
- Kitamura Y, Scavarda N, Wells SA, et al.: Two maternally derived missense mutations in the tyrosine kinase domain of the RET protooncogene in a patient with de novo MEN 2B. Hum Mol Genet 4 (10): 1987-8, 1995. [PUBMED Abstract]
Genotype-Phenotype Correlations and Risk Stratification in Multiple Endocrine Neoplasia Type 2 (MEN2)
Genotype-phenotype correlations in MEN2 are well established and have long been used to guide clinicians in making medical management recommendations. Several groups have developed pathogenic variant–stratification tables based on clinical phenotype, age of onset, and aggressiveness of medullary thyroid cancer (MTC).[1-3] This classification strategy was first put forth after the Seventh International Workshop on MEN in 2001, which provided guidelines for the age of genetic testing and risk-reducing thyroidectomy.[1] This stratification has been revised by the American Thyroid Association (ATA).[3-5] The specific pathogenic variants and their ATA classification are summarized in Table 1 below.
ATA-Highest Risk (HST) RET pathogenic variants are the most aggressive and carry the highest risk of developing MTC.[3] This category includes those with multiple endocrine neoplasia type 2B (MEN2B) and RET codon M918T pathogenic variants and is associated with the youngest age at disease onset and the highest risk of mortality. ATA-High Risk (H) RET pathogenic variants, at codons 634 and 883, are associated with a slightly lower risk, yet the MTC in patients with these pathogenic variants is still quite aggressive and may present at an early age.[6] The third category of RET variants are designated as ATA-Moderate Risk (MOD) variants.[5] These variants are associated with lower risk of aggressive MTC relative to the risk seen in carriers of ATA-HST and ATA-H RET pathogenic variants. Results from a study of 387 RET pathogenic variant carriers with MTC have suggested that ATA-MOD variants may be associated with MTC as aggressive as seen in individuals with ATA-H variants but present at a later age.[7] The risk of MTC is still substantially elevated over the general population risk, and consideration of risk-reducing thyroidectomy is warranted.[3] Patients with early stage I and stage II disease can achieve 100% survival rates regardless of the ATA risk category.[7] Common pathogenic variants in the ATA-MOD category are shown in Table 1.
Pathogenic variants at codons 883 and 918 have been seen only in MEN2B and are associated with the earliest age of onset and the most aggressive form of MTC.[6,8-12] Approximately 95% of individuals with MEN2B have the M918T pathogenic variant.[8-10,13] As discussed above, 50% of individuals with MEN2B will develop pheochromocytoma (PHEO), but primary hyperparathyroidism (PHPT) is rare. A retrospective review of all published cases of A883F variant carriers (N = 13) found that the MTC disease course was more indolent than what was observed in M918T carriers. A883F carriers had later disease onset (50% penetrance for MTC at age 19 y), 5- and 10-year survival rates of 88%, and 63% of patients achieved biochemical cure for MTC.[6] In addition to variants at codons 883 and 918, some individuals with an MEN2B-like phenotype have been found to carry two germline variants on the same allele.[14-18] It is likely that as testing for RET becomes more common in clinical practice, additional double variant phenotypes will be described.
Pathogenic variants at codon 634 (ATA-H) are by far the most frequent finding in families with multiple endocrine neoplasia type 2A (MEN2A). One study of 477 RET carriers showed that 52.1% had the C634R pathogenic variant, 26.0% carried the C634Y pathogenic variant, and 9.1% had the C634G pathogenic variant.[19] In general, pathogenic variants in codon 634 are associated with PHEOs and PHPT.[19,20] MEN2A with cutaneous lichen amyloidosis had been seen almost exclusively in patients with pathogenic variants in codon 634, although MTC and cutaneous lichen amyloidosis have been reported in a patient with a pathogenic variant in codon 804.[19,21-23] Codon 634 pathogenic variants have also been described in familial medullary thyroid cancer (FMTC) but are almost exclusively C634Y.[19]
Moderate-risk variants located in exon 10 of the RET gene include variants in codons 609, 611, 618, 620, and 630. These variants involve cysteine residues in the extracellular domain of the RET protein and have been seen in families with MEN2A and those with MTC only (FMTC).[2,19,24-29] The risk of MTC in individuals with these pathogenic variants can be as high as 95%. The risk of PHEO and hyperparathyroidism is lower than that seen in individuals with high-risk pathogenic variants.
Individuals with pathogenic variants in codons 321, 515, 533, 600, 603, 606, 531/9 base pair duplication, and 532 duplication have a lower, albeit still elevated, lifetime risk of MTC. MTC associated with these pathogenic variants tends to follow a more indolent course and have a later age at onset, although there are several reports of individuals with these pathogenic variants who developed MTC before age 20 years.[19,30-34] Although PHEO and PHPT are not commonly associated with these pathogenic variants, they have been described.[34]
| RET Pathogenic variant | Exon | Risk of Aggressive MTC | Approximate Incidence of PHEO | Approximate Incidence of PHPT | Presence of CLA | Presence of HSCR |
|---|---|---|---|---|---|---|
| CLA = cutaneous lichen amyloidosis; HSCR = Hirschsprung disease; MTC = medullary thyroid cancer; PHEO = pheochromocytoma; PHPT = primary hyperparathyroidism. | ||||||
| aAdapted from Wells et al.[3] | ||||||
| G533C | 8 | Moderate | 10% | - | N | N |
| C609F/G/R/S/Y | 10 | Moderate | 10%–30% | 10% | N | Y |
| C611F/G/S/Y/W | 10 | Moderate | 10%–30% | 10% | N | Y |
| C618F/R/S | 10 | Moderate | 10%–30% | 10% | N | Y |
| C620F/R/S | 10 | Moderate | 10%–30% | 10% | N | Y |
| C630R/Y | 11 | Moderate | 10%–30% | 10% | N | N |
| D631Y | 11 | Moderate | 50% | - | N | N |
| C634F/G/R/S/W/Y | 11 | High | 50% | 20%–30% | Y | N |
| K666E | 11 | Moderate | 10% | - | N | N |
| E768D | 13 | Moderate | - | - | N | N |
| L790F | 13 | Moderate | 10% | - | N | N |
| V804L | 14 | Moderate | 10% | 10% | N | N |
| V804M | 14 | Moderate | 10% | 10% | Y | N |
| A883F | 15 | High | 50% | - | N | N |
| S891A | 15 | Moderate | 10% | 10% | N | N |
| R912P | 16 | Moderate | - | - | N | N |
| M918T | 16 | Highest | 50% | - | N | N |
In addition to the pathogenic variants categorized in Table 1, a number of rare or novel RET variants have been described. Some of these represent pathogenic variants that lead to MEN2A phenotypes. Others may represent low-penetrance alleles or modifying alleles that confer only a modest risk of developing MTC.[35] A multicenter study identified eight families with a RET K666N variant. Of the 16 screened family members identified as having a pathogenic variant, only one had MTC.[35] Still others may have benign polymorphisms of no clinical significance. For example, some studies show compelling evidence that RET variants Y791F (p.Tyr791Phe) and S649L (p.Ser649Leu) are likely benign polymorphisms; this was based on the equal frequencies seen between cases and healthy controls and co-occurrence with other disease-causing variants that cosegregate with disease in the family.[36,37] A long-term follow-up study of Danish Y791F carriers (n = 20) showed no sign of MEN2A (MTC, PHPT, PHEO, cutaneous lichen amyloidosis, or Hirschsprung disease) among the cohort, with a median age of 49.5 years (range, 7–87 y).[38] Therefore, carriers of these variants are not followed with MEN2 management guidelines. Asymptomatic family members are generally not tested for these variants.
Research is ongoing into the role of neutral RET sequence variants in modifying the clinical presentation of patients with MEN2A. The presence of certain RET polymorphisms or haplotypes is being analyzed for its impact on the likelihood for development of PHEO, hyperparathyroidism, HSCR, and age at onset of metastatic involvement with MTC.[39-42] A variety of approaches, including segregation analyses, in silico analyses, association studies, and functional assays, can be employed to determine the functional and clinical significance of a given genetic variant.[43]
References
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PUBMED Abstract]
- Kouvaraki MA, Shapiro SE, Perrier ND, et al.: RET proto-oncogene: a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid 15 (6): 531-44, 2005. [PUBMED Abstract]
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015. [PUBMED Abstract]
- Kloos RT, Eng C, Evans DB, et al.: Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19 (6): 565-612, 2009. [PUBMED Abstract]
- Voss RK, Feng L, Lee JE, et al.: Medullary Thyroid Carcinoma in MEN2A: ATA Moderate- or High-Risk RET Mutations Do Not Predict Disease Aggressiveness. J Clin Endocrinol Metab 102 (8): 2807-2813, 2017. [PUBMED Abstract]
- Mathiesen JS, Habra MA, Bassett JHD, et al.: Risk Profile of the RET A883F Germline Mutation: An International Collaborative Study. J Clin Endocrinol Metab 102 (6): 2069-2074, 2017. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Lymph node metastasis in hereditary medullary thyroid cancer is independent of the underlying RET germline mutation. Eur J Surg Oncol 47 (4): 920-923, 2021. [PUBMED Abstract]
- Eng C, Smith DP, Mulligan LM, et al.: Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet 3 (2): 237-41, 1994. [PUBMED Abstract]
- Hofstra RM, Landsvater RM, Ceccherini I, et al.: A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367 (6461): 375-6, 1994. [PUBMED Abstract]
- Carlson KM, Dou S, Chi D, et al.: Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A 91 (4): 1579-83, 1994. [PUBMED Abstract]
- Gimm O, Marsh DJ, Andrew SD, et al.: Germline dinucleotide mutation in codon 883 of the RET proto-oncogene in multiple endocrine neoplasia type 2B without codon 918 mutation. J Clin Endocrinol Metab 82 (11): 3902-4, 1997. [PUBMED Abstract]
- Smith DP, Houghton C, Ponder BA: Germline mutation of RET codon 883 in two cases of de novo MEN 2B. Oncogene 15 (10): 1213-7, 1997. [PUBMED Abstract]
- Eng C, Mulligan LM, Healey CS, et al.: Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res 56 (9): 2167-70, 1996. [PUBMED Abstract]
- Cranston AN, Carniti C, Oakhill K, et al.: RET is constitutively activated by novel tandem mutations that alter the active site resulting in multiple endocrine neoplasia type 2B. Cancer Res 66 (20): 10179-87, 2006. [PUBMED Abstract]
- Miyauchi A, Futami H, Hai N, et al.: Two germline missense mutations at codons 804 and 806 of the RET proto-oncogene in the same allele in a patient with multiple endocrine neoplasia type 2B without codon 918 mutation. Jpn J Cancer Res 90 (1): 1-5, 1999. [PUBMED Abstract]
- Kameyama K, Okinaga H, Takami H: RET oncogene mutations in 75 cases of familial medullary thyroid carcinoma in Japan. Biomed Pharmacother 58 (6-7): 345-7, 2004 Jul-Aug. [PUBMED Abstract]
- Iwashita T, Murakami H, Kurokawa K, et al.: A two-hit model for development of multiple endocrine neoplasia type 2B by RET mutations. Biochem Biophys Res Commun 268 (3): 804-8, 2000. [PUBMED Abstract]
- Menko FH, van der Luijt RB, de Valk IA, et al.: Atypical MEN type 2B associated with two germline RET mutations on the same allele not involving codon 918. J Clin Endocrinol Metab 87 (1): 393-7, 2002. [PUBMED Abstract]
- Eng C, Clayton D, Schuffenecker I, et al.: The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 276 (19): 1575-9, 1996. [PUBMED Abstract]
- Mulligan LM, Eng C, Healey CS, et al.: Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet 6 (1): 70-4, 1994. [PUBMED Abstract]
- Seri M, Celli I, Betsos N, et al.: A Cys634Gly substitution of the RET proto-oncogene in a family with recurrence of multiple endocrine neoplasia type 2A and cutaneous lichen amyloidosis. Clin Genet 51 (2): 86-90, 1997. [PUBMED Abstract]
- Yip L, Cote GJ, Shapiro SE, et al.: Multiple endocrine neoplasia type 2: evaluation of the genotype-phenotype relationship. Arch Surg 138 (4): 409-16; discussion 416, 2003. [PUBMED Abstract]
- Rothberg AE, Raymond VM, Gruber SB, et al.: Familial medullary thyroid carcinoma associated with cutaneous lichen amyloidosis. Thyroid 19 (6): 651-5, 2009. [PUBMED Abstract]
- Eng C, Mulligan LM, Smith DP, et al.: Low frequency of germline mutations in the RET proto-oncogene in patients with apparently sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 43 (1): 123-7, 1995. [PUBMED Abstract]
- Bolino A, Schuffenecker I, Luo Y, et al.: RET mutations in exons 13 and 14 of FMTC patients. Oncogene 10 (12): 2415-9, 1995. [PUBMED Abstract]
- Boccia LM, Green JS, Joyce C, et al.: Mutation of RET codon 768 is associated with the FMTC phenotype. Clin Genet 51 (2): 81-5, 1997. [PUBMED Abstract]
- Lesueur F, Cebrian A, Cranston A, et al.: Germline homozygous mutations at codon 804 in the RET protooncogene in medullary thyroid carcinoma/multiple endocrine neoplasia type 2A patients. J Clin Endocrinol Metab 90 (6): 3454-7, 2005. [PUBMED Abstract]
- Shannon KE, Gimm O, Hinze R: Germline V804M mutation in the RET protooncogene in 2 apparently sporadic cases of MTC presenting in the 7th decade of life. The Journal of Endocrine Genetics 1 (1): 39-46, 1999.
- Raue F, Frank-Raue K: Genotype-phenotype relationship in multiple endocrine neoplasia type 2. Implications for clinical management. Hormones (Athens) 8 (1): 23-8, 2009 Jan-Mar. [PUBMED Abstract]
- Mulligan LM, Marsh DJ, Robinson BG, et al.: Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the International RET Mutation Consortium. J Intern Med 238 (4): 343-6, 1995. [PUBMED Abstract]
- Moers AM, Landsvater RM, Schaap C, et al.: Familial medullary thyroid carcinoma: not a distinct entity? Genotype-phenotype correlation in a large family. Am J Med 101 (6): 635-41, 1996. [PUBMED Abstract]
- Niccoli-Sire P, Murat A, Rohmer V, et al.: Familial medullary thyroid carcinoma with noncysteine ret mutations: phenotype-genotype relationship in a large series of patients. J Clin Endocrinol Metab 86 (8): 3746-53, 2001. [PUBMED Abstract]
- Machens A, Ukkat J, Brauckhoff M, et al.: Advances in the management of hereditary medullary thyroid cancer. J Intern Med 257 (1): 50-9, 2005. [PUBMED Abstract]
- Mukherjee S, Zakalik D: RET codon 804 mutations in multiple endocrine neoplasia 2: genotype-phenotype correlations and implications in clinical management. Clin Genet 79 (1): 1-16, 2011. [PUBMED Abstract]
- Xu JY, Grubbs EG, Waguespack SG, et al.: Medullary Thyroid Carcinoma Associated with Germline RETK666N Mutation. Thyroid 26 (12): 1744-1751, 2016. [PUBMED Abstract]
- Erlic Z, Hoffmann MM, Sullivan M, et al.: Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel-Lindau syndrome. J Clin Endocrinol Metab 95 (1): 308-13, 2010. [PUBMED Abstract]
- Toledo RA, Hatakana R, Lourenço DM, et al.: Comprehensive assessment of the disputed RET Y791F variant shows no association with medullary thyroid carcinoma susceptibility. Endocr Relat Cancer 22 (1): 65-76, 2015. [PUBMED Abstract]
- Høxbroe Michaelsen S, Ornstrup MJ, Poulsen MM, et al.: Long-term follow-up of RET Y791F carriers in Denmark 1994-2017: A National Cohort Study. J Surg Oncol 119 (6): 687-693, 2019. [PUBMED Abstract]
- Siqueira DR, Ceolin L, Ferreira CV, et al.: Role of RET genetic variants in MEN2-associated pheochromocytoma. Eur J Endocrinol 170 (6): 821-8, 2014. [PUBMED Abstract]
- Ceolin L, Siqueira DR, Romitti M, et al.: Molecular basis of medullary thyroid carcinoma: the role of RET polymorphisms. Int J Mol Sci 13 (1): 221-39, 2012. [PUBMED Abstract]
- Robledo M, Gil L, Pollán M, et al.: Polymorphisms G691S/S904S of RET as genetic modifiers of MEN 2A. Cancer Res 63 (8): 1814-7, 2003. [PUBMED Abstract]
- Kaczmarek-Ryś M, Ziemnicka K, Pławski A, et al.: Modifying impact of RET gene haplotypes on medullary thyroid carcinoma clinical course. Endocr Relat Cancer 25 (4): 421-436, 2018. [PUBMED Abstract]
- Margraf RL, Crockett DK, Krautscheid PM, et al.: Multiple endocrine neoplasia type 2 RET protooncogene database: repository of MEN2-associated RET sequence variation and reference for genotype/phenotype correlations. Hum Mutat 30 (4): 548-56, 2009. [PUBMED Abstract]
Screening and Surveillance in Multiple Endocrine Neoplasia Type 2 (MEN2)
Screening and Surveillance for Pheochromocytomas (PHEOs)
The presence of a functioning PHEO can be excluded by appropriate biochemical screening before thyroidectomy in any patient with multiple endocrine neoplasia type 2A (MEN2A) or multiple endocrine neoplasia type 2B (MEN2B). However, childhood PHEOs are rare in MEN2.[1] Individuals with American Thyroid Association (ATA)-Highest Risk (HST) or ATA-High Risk (H) RET pathogenic variants have an increased risk of PHEO (up to 50%).[2] The ATA recommends that annual screening for PHEO be considered by age 11 years in patients with ATA-HST or ATA-H RET pathogenic variants.[1] The ATA recommends that patients with ATA-Moderate Risk (MOD) RET pathologic variants have periodic screening for PHEO beginning by age 16 years.[1] Magnetic resonance imaging or other imaging tests may be ordered only if the biochemical results are abnormal.[3-5] Studies of individuals with sporadic or hereditary PHEO (including, but not limited to, individuals with MEN2) have suggested that measurement of catecholamine metabolites, specifically plasma-free metanephrines and/or urinary fractionated metanephrines, provides a higher diagnostic sensitivity than urinary catecholamines because of the episodic nature of catecholamine excretion.[5-14] Several reviews provide a succinct summary of the biochemical diagnosis, localization, and management of PHEO.[6,15]
Screening and Surveillance for Hyperparathyroidism
Primary hyperparathyroidism is variably reported in MEN2A, with rates ranging from 2% to 35%.[16] However, large, multicenter studies suggest rates in the lower half of this range. MEN2-related hyperparathyroidism is generally associated with mild, often asymptomatic hypercalcemia early in the natural history of the disease, which, left untreated, may become symptomatic.[17] Those with higher risk pathogenic variants are more likely to develop hyperparathyroidism, with an earlier age of onset.[16] Childhood hyperparathyroidism is rare in MEN2. Four studies reported a median age of diagnosis between ages 38 and 45 years.[16-19] The ATA provides recommendations for annual screening for hyperparathyroidism,[1] with screening beginning by age 11 years in carriers of ATA-HST and ATA-H pathogenic variants and by age 16 years for carriers of ATA-MOD RET pathogenic variants.[1] Testing typically includes albumin-corrected calcium or ionized serum calcium, with or without intact parathyroid hormone measurement. While cure rates for hyperparathyroidism are high, recurrence can occur decades after initial treatment.[16]
References
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015. [PUBMED Abstract]
- Quayle FJ, Fialkowski EA, Benveniste R, et al.: Pheochromocytoma penetrance varies by RET mutation in MEN 2A. Surgery 142 (6): 800-5; discussion 805.e1, 2007. [PUBMED Abstract]
- Modigliani E, Vasen HM, Raue K, et al.: Pheochromocytoma in multiple endocrine neoplasia type 2: European study. The Euromen Study Group. J Intern Med 238 (4): 363-7, 1995. [PUBMED Abstract]
- Wells SA, Donis-Keller H: Current perspectives on the diagnosis and management of patients with multiple endocrine neoplasia type 2 syndromes. Endocrinol Metab Clin North Am 23 (1): 215-28, 1994. [PUBMED Abstract]
- Lenders JW, Duh QY, Eisenhofer G, et al.: Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99 (6): 1915-42, 2014. [PUBMED Abstract]
- Pacak K, Eisenhofer G, Ahlman H, et al.: Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab 3 (2): 92-102, 2007. [PUBMED Abstract]
- Gardet V, Gatta B, Simonnet G, et al.: Lessons from an unpleasant surprise: a biochemical strategy for the diagnosis of pheochromocytoma. J Hypertens 19 (6): 1029-35, 2001. [PUBMED Abstract]
- Gerlo EA, Sevens C: Urinary and plasma catecholamines and urinary catecholamine metabolites in pheochromocytoma: diagnostic value in 19 cases. Clin Chem 40 (2): 250-6, 1994. [PUBMED Abstract]
- Guller U, Turek J, Eubanks S, et al.: Detecting pheochromocytoma: defining the most sensitive test. Ann Surg 243 (1): 102-7, 2006. [PUBMED Abstract]
- Lenders JW, Pacak K, Walther MM, et al.: Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287 (11): 1427-34, 2002. [PUBMED Abstract]
- Raber W, Raffesberg W, Bischof M, et al.: Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med 160 (19): 2957-63, 2000. [PUBMED Abstract]
- Sawka AM, Jaeschke R, Singh RJ, et al.: A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88 (2): 553-8, 2003. [PUBMED Abstract]
- Unger N, Pitt C, Schmidt IL, et al.: Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol 154 (3): 409-17, 2006. [PUBMED Abstract]
- Därr R, Kuhn M, Bode C, et al.: Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine 56 (3): 495-503, 2017. [PUBMED Abstract]
- Pacak K, Ilias I, Adams KT, et al.: Biochemical diagnosis, localization and management of pheochromocytoma: focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. J Intern Med 257 (1): 60-8, 2005. [PUBMED Abstract]
- Holm M, Vestergaard P, Poulsen MM, et al.: Primary Hyperparathyroidism in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Population-Based Retrospective Study. Cancers (Basel) 15 (7): , 2023. [PUBMED Abstract]
- Kraimps JL, Denizot A, Carnaille B, et al.: Primary hyperparathyroidism in multiple endocrine neoplasia type IIa: retrospective French multicentric study. Groupe d'Etude des Tumeurs á Calcitonine (GETC, French Calcitonin Tumors Study Group), French Association of Endocrine Surgeons. World J Surg 20 (7): 808-12; discussion 812-3, 1996. [PUBMED Abstract]
- Raue F, Kraimps JL, Dralle H, et al.: Primary hyperparathyroidism in multiple endocrine neoplasia type 2A. J Intern Med 238 (4): 369-73, 1995. [PUBMED Abstract]
- Milos IN, Frank-Raue K, Wohllk N, et al.: Age-related neoplastic risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germ line RET Cys634Trp (TGC>TGG) mutation. Endocr Relat Cancer 15 (4): 1035-41, 2008. [PUBMED Abstract]
Interventions in Multiple Endocrine Neoplasia Type 2 (MEN2)
Risk-Reducing Thyroidectomy
For more information about risk stratification, see the Genotype-Phenotype Correlations and Risk Stratification in MEN2 section.
Risk-reducing thyroidectomy (also referred to as early thyroidectomy and previously referred to as prophylactic thyroidectomy) is the oncological treatment of choice for patients with MEN2. Children with the M918T RET pathogenic variant may benefit from a thyroidectomy in the first year of life, perhaps in the first months of life.[1] Likewise, children with American Thyroid Association (ATA)-High Risk (H) category RET pathogenic variants may undergo risk-reducing thyroidectomy around age 5 years or earlier, based on serum calcitonin levels. The ATA recommends that children in the ATA-Moderate Risk (MOD) category have a physical examination, ultrasonography of the neck, and measurement of serum calcitonin beginning around age 5 years, as these tumors may have later onset but are similarly aggressive once this is taken into account.[1,2] The absence of an abnormal calcitonin level may prompt continued measurement every 6 to 12 months.
A multidisciplinary team caring for the patient, including the pediatrician, pediatric endocrinologist, and surgeon should determine the timing of surgery in conjunction with the child’s parents based on the trend in serum calcitonin levels, ultrasonographic findings, preference of the family, and experience of the treating physicians.[1]
In children with some ATA-H or ATA-MOD RET pathogenic variants, earlier studies have suggested that basal and pentagastrin-stimulated calcitonin levels could be used to determine the timing of total thyroidectomy.[3-6] These findings suggest that surgery may be safely delayed in carriers of an RET pathogenic variant until basal or stimulated calcitonin levels increase on routine testing. The benefits of this approach are particularly noteworthy in the younger population of pathogenic variant carriers, as a delay in surgery until the patient is older may reduce the risk of surgical complications. However, pentagastrin is not readily available. A large study of 2,740 children aged 16 years and under has provided data on age-specific reference ranges for calcitonin levels in younger children that may assist in decision making.[7] Because some calcitonin assays may be more sensitive than others,[5] attention to the type of testing, as well as calcitonin levels must be considered. However, normal preoperative basal calcitonin does not exclude the possibility of the patient having medullary thyroid cancer (MTC).[8]
For patients with RET germline variants, older age at risk-reducing thyroidectomy has been significantly associated with a higher risk of persistent or recurrent disease.[9,10] Even micro MTC carries a risk of biochemical recurrence.[10] Consistent with this finding, a study of young, clinically asymptomatic individuals with multiple endocrine neoplasia type 2A (MEN2A) found a lower incidence of persistent or recurrent disease in patients who had thyroidectomy earlier in life (younger than age 8 y) and who had no evidence of lymph node metastases.[11] Several studies have found a significantly lower rate of invasive or metastatic MTC among those who undergo surgery at an earlier age than among those who undergo surgery at a later age.[12] For patients with the most aggressive M918T RET variant, cure is exceptional if surgery is performed after age 4 years.[3,13] Together, these findings are consistent with more favorable outcomes for patients undergoing risk-reducing thyroidectomy at a young age.[14]
While performing thyroidectomy before biochemical evidence of disease exists (normal preoperative calcitonin) may reduce the risk of recurrent disease, postoperative and lifelong surveillance strategies are often needed. These strategies may depend on the final pathological findings (if carcinoma was present and whether it was microscopic disease or macroscopic disease).[1,15] One study found that 10% of patients with MEN2A undergoing thyroidectomy developed recurrent disease, based on initially undetectable basal and stimulated calcitonin levels (<2 pg/mL) that became positive 5 to 10 years after surgery.[11] Only 2% of patients had residual disease after risk-reducing surgery as assessed by a persistently elevated basal or stimulated calcitonin.[11] Since screening and surveillance in second-generation pediatric patients with multiple endocrine neoplasia type 2B (MEN2B) begin earlier, timing of surgical intervention has decreased the incidence of lymph node metastases. This finding encourages the role of personalized extent of surgery intervention and thyroidectomy alone, without ipsilateral central neck dissection can be adequate for cure in 97% of patients.[16]
Age at MTC diagnosis is variable. Reports have documented MTC metastasis in MEN2B cases before age 3 years and in MEN2A cases with ATA-H or ATA-MOD RET variants before age 6 years.[3,11,13,17] Conversely, some families with the familial medullary thyroid cancer subtype of MEN2A did not show signs of disease until midlife. In addition, some elderly relatives who carried the RET variant never developed MTC.[18] Additional data have suggested that some ATA-MOD RET variants (which were previously thought to be more indolent) may be as aggressive as ATA-H RET variants, but they are associated with delayed disease onset.[2,19]
Treatment for MEN2-Related Medullary Thyroid Cancer (MTC)
For general information about MTC treatment, see the MTC section in Thyroid Cancer Treatment. For more information about treatment for children with MEN2, see the Childhood MEN Syndromes Treatment summary.
Therapeutic thyroidectomy
The standard treatment for adults with MTC is surgical removal of the entire thyroid gland, including the posterior capsule and central lymph node dissection.[1] A therapeutic central neck dissection is typically performed if there is radiographic evidence of metastatic lymph node involvement or if the serum calcitonin level is higher than 40 pg/mL.[1] The decision to perform a risk-reducing central neck dissection is generally made based on multiple factors such as patient age, pathogenic variant, presence of concomitant primary hyperparathyroidism (PHPT), and the viability of in situ parathyroid glands.[1] Selective autotransplantation of parathyroid glands that were devascularized during a risk-reducing thyroidectomy and/or central neck clearance is recommended, but risk-reducing autotransplantation is not suggested.[1] A selective approach also significantly reduces the detrimental outcome of hypoparathyroidism.[20]
The MEN2B RET variant M918T is associated with approximately 100% incidence of MTC in the first years of life [13] and is considered the most aggressive MEN2 phenotype. In patients with MEN2A, the ATA-H high-risk codon 634 pathogenic variant is much more likely to be associated with invasive or metastatic MTC and development of persistent or recurrent disease than pathogenic variants in codons 804, 618, or 620.[12] One series of 503 at-risk individuals with ATA-MOD category pathogenic variants (including codons 533, 609, 611, 618, 620, 791, and 804) reported cumulative penetrance rates, median time to MTC, and positive predictive value of preoperative calcitonin.[4] The risk of developing MTC by age 50 years ranged from 18% to 95%, depending on the codon, with codon 620 having the highest penetrance. Most patients with MTC had node-negative disease, confirming the more indolent disease course previously reported with these pathogenic variants. Although an elevated preoperative calcitonin level strongly predicted the presence of MTC, relatively high false-negative rates (low normal calcitonin levels with MTC) were noted for many of the codons. This information is useful when counseling carriers of pathogenic variants regarding the extent of surgical resection.
The ATA recommends compartment-directed lymph node dissection for local or regional disease (no evidence of distant metastases) in the following situations:[1]
- If there is no evidence of neck nodal metastases by ultrasonography in biopsy-proven thyroid disease, risk-reducing central neck dissection should be performed concomitant with initial thyroidectomy.
- If nodal disease is present in either the central or lateral neck, a compartment-oriented lateral neck dissection of the ipsilateral side should be performed.
- If nodal disease is present and basal calcitonin levels are greater than 200 pg/mL, then consider contralateral lateral neck dissection.
Although basal calcitonin levels may not be able to identify all patients with MTC preoperatively, this test has utility as a predictor of postoperative remission, lymph node metastases, and distant metastases.[21] In one study of 224 patients from a single institution, preoperative basal calcitonin levels greater than 500 pg/mL predicted failure to achieve biochemical remission.[21] The authors of this study found that nodal metastases started appearing at basal calcitonin levels of 40 pg/mL (normal, <10 pg/mL). In node-positive patients, distant metastases emerged at basal calcitonin levels of 150 pg/mL to 400 pg/mL. Another study of 308 RET pathogenic variant carriers found that a normal basal preoperative calcitonin excluded the presence of lymph node metastases (negative predictive value, 100%).[6] Therefore, the preoperative basal calcitonin level is a useful prognostic indicator and may help guide the surgical approach.
Level of evidence (therapeutic thyroidectomy): 3dii
Level of evidence (central neck dissection): 4
Prognosis
Structural and metastatic MTC recurrence is common in germline RET carriers. Recurrence can happen up to 20 years after initial treatment. However, overall survival (OS) is generally favorable, with one study citing an OS rate of 92% after 10 years.[22]
Hormone replacement therapy after total thyroidectomy
Patients who have had total thyroidectomy require lifelong thyroid hormone replacement therapy. Medication dosing is age-dependent, and treatment may be initiated based on ideal body weight. For healthy adults aged 60 years and younger (without cardiac disease), a reasonable starting dose is 1.6 µg/kg to 1.8 µg/kg, given once daily.[23] Older patients may require 20% to 30% less thyroid hormone than younger patients.[24] Children metabolize thyroxine (T4) more rapidly than adults and require higher replacement by body weight. Depending on the age of the child, the amount of T4 replacement can range from 2 µg/kg to 6 µg/kg.[25] However, T4 replacement is preferred over suppressive therapy. Since C-cell tumors are not dependent on thyroid-stimulating hormone (TSH) to grow, T4 therapy for MTC patients may be adjusted to maintain TSH levels that are within the normal reference range. Thyroglobulin measurements may be useful to help adjust and maintain TSH levels within a normal reference range. This can prevent regrowth of remnant thyroid tissue.[26]
Adjuvant therapy for MTC
Chemotherapy and radiation therapy are generally not effective against MTC.[27-29] Targeted molecular therapies are being explored to manage MTC. RET inhibitors and multikinase inhibitors are being used to block RET activity.
Two U.S. Food and Drug Administration (FDA)–approved RET inhibitors (pralsetinib and selpercatinib) are available for patients with MTC who have a RET single nucleotide variant. These RET inhibitors are also available for patients who have differentiated thyroid cancers with a RET fusion. A multicenter, phase I/II trial (ARROW) was conducted to evaluate the efficacy of pralsetinib in patients with RET-mutant MTC with or without prior treatment with vandetanib or cabozantinib. Among 55 patients who were previously treated with a multikinase inhibitor, the overall response rate was 60% (95% confidence interval [CI], 46%–73%) and the 1-year progression-free survival (PFS) rate was 75% (95% CI, 63%–86%). Among 21 treatment-naïve patients, the overall response rate was 71% (95% CI, 48%–89%) and the 1-year PFS rate was 81% (95% CI, 63%–98%).[30] A similar phase I/II trial (LIBRETTO) examined the efficacy of selpercatinib in patients with RET-mutant MTC with or without prior treatment with vandetanib or cabozantinib. Among 55 patients who were previously treated with a multikinase inhibitor, 69% had an objective response (95% CI, 55%–81%); the 1-year PFS rate was 82% (95% CI, 69%–90%). Among 88 treatment-naïve patients, the objective response rate was 73% (95% CI, 62%–82%), and the 1-year PFS rate was 92% (95% CI, 82%–97%).[31]
The use of vandetanib and cabozantinib are FDA-approved for adult patients with progressive metastatic MTC who are ineligible for surgery. A phase III study found that PFS was longer in adults who received vandetanib than in those who received placebo.[32] A phase I/phase II study of children with MEN2B found an objective partial response rate of 47% with vandetanib.[33] Subsequent follow-up analysis of this cohort revealed that a partial response was seen in 10 of 17 patients; stable disease was seen in an additional 6 individuals. Median PFS was 6.7 years.[34] A double-blind phase III trial that compared cabozantinib with placebo in 330 patients with progressive MTC showed an improvement in median PFS across all subgroups.[35,36] In this trial, patients who had pathogenic variants, including RET or RAS, were more likely to have a prolonged PFS compared with patients lacking both pathogenic variants.[37] Prospective studies may further clarify whether particular pathogenic variants can be used to guide therapy. Neither cabozantinib nor vandetanib has demonstrated improved OS.[32,35,36] Importantly, these agents are not effective at inhibiting some MEN2 RET variants, specifically those at codon 804,[38] making genotype an important consideration for treatment with RET inhibitors. Further, a 2018 study demonstrated the development of resistance to these agents through somatic acquisition of a V804M variant in RET.[39] Finally, multikinase inhibitors are associated with significant toxicities, possibly due to their off-target effects on other kinases.[40] Other multikinase inhibitors for targeting RET are being studied in clinical trials; however, they may provide only limited advantages over vandetanib and cabozantinib. For these reasons, ongoing studies are focusing on the development of selective RET inhibitors with fewer off-target effects that are able to block the activity of all RET variant forms and the use of combination therapy in MTC. Future studies will likely focus on the development of new targeted therapies and the use of combination therapy in MTC.[41,42]
Level of evidence (pralsetinib): 4
Level of evidence (selpercatinib): 3dii
Level of evidence (vandetanib): 2
Level of evidence (cabozantinib): 1
For more information, see Thyroid Cancer Treatment.
Treatment for MEN2-Related Pheochromocytoma (PHEO)
A cognitive shift has occurred in the field regarding the risks and benefits of whole organ resection. This shift is especially relevant for endocrine glands that are difficult to manage postresection and may require replacement therapy. PHEO may be either unilateral or bilateral in patients with MEN2. Laparoscopic adrenalectomy (anterior or posterior) is the recommended approach after appropriate preoperative medical blockade for the treatment of unilateral PHEO.[1,43-45] The risks, benefits, and potential of life-threatening adrenal insufficiency should be considered at the time of the initial operative planning.
If disease appears unilateral, the contralateral gland may develop metachronous disease in 17% to 72% of patients.[46,47] In a series of RET p.Cys634 variant carriers, only 1 in 5 developed a second ipsilateral PHEO after a follow-up period of 8 to 11 years when the cortical preservation technique was used. This technique allows patients to continue making steroid hormones on their own, which makes it a viable surgical option.[48] In one series, 23 patients with a unilateral PHEO and a macroscopically normal contralateral adrenal gland were treated initially with unilateral adrenalectomy.[49] A PHEO developed within the retained gland in 12 (52%) of these patients, occurring a mean of 11.9 years after initial surgery. During follow-up, no patient experienced a hypertensive crisis or other problems attributable to an undiagnosed PHEO. In contrast, 10 of 43 patients (23%) treated with bilateral adrenalectomy experienced at least one episode of acute adrenal insufficiency. Thus, unilateral adrenalectomy is a reasonable management strategy for unilateral PHEO in patients with MEN2.[1,50,51] Many experts suggest considering a cortical-sparing technique for a suspected unilateral PHEO, even if it is the patient's first operation.[1,52] For more information, see the section on Interventions in Genetics of Endocrine and Neuroendocrine Neoplasias. Because of the risk of contralateral gland disease, periodic surveillance (serum or urinary catecholamine measurements) for the development of disease in the contralateral adrenal gland is recommended.[1]
Regarding the operative approach, several studies found posterior retroperitoneoscopic adrenalectomy to be safe and effective, with very low mortality and a low rate of minor complications. Conversion to open surgery was rarely required.[46,53-59]
There are other clinical situations (besides surgery) in which patients with catecholamine excess face special risks. An example is the healthy at-risk female patient who becomes pregnant.[60] Pregnancy, labor, and delivery can prompt a hypertensive crisis in individuals with an undetected PHEO. Pregnant patients who are found to have catecholamine excess require pharmacotherapy before delivery.[61]
Treatment for MEN2-Related Hyperparathyroidism
Most patients with MEN2-related parathyroid disease are either asymptomatic or diagnosed incidentally during preoperative planning or at the time of thyroidectomy. Typically, hypercalcemia (when present) is mild, although it may be associated with increased urinary excretion of calcium and nephrolithiasis. As a consequence, the indications for surgical intervention are generally similar to those recommended for patients with sporadic PHPT.[43] In general, fewer than four of the parathyroid glands are involved at the time of detected abnormalities in calcium metabolism.[1]
Treatment of hyperparathyroidism typically employs some surgical removal of the involved glands. Cure of hyperparathyroidism was achieved surgically in 89% of one large series of MEN2A patients;[62] however, 22% of resected patients in this study developed postoperative hypoparathyroidism. Five patients (9%) had recurrent hyperparathyroidism. This series used various surgical techniques, including total parathyroidectomy with autotransplantation to the nondominant forearm (4 of 11 patients [36%] developed postoperative hypoparathyroidism), subtotal parathyroidectomy (6 of 12 patients [50%] developed hypoparathyroidism), and resection only of glands that were macroscopically enlarged (3 of 29 patients [10%] developed hypoparathyroidism). These data indicate that excision of only parathyroid glands that are enlarged appears to be sufficient therapy in most cases.
Some investigators have suggested using the MEN2 subtype to decide where to place the parathyroid glands that are identified at the time of thyroid surgery. For patients with MEN2B in whom the risk of parathyroid disease is quite low, the parathyroid glands may be left in situ in the neck. For adult patients with MEN2A, in whom the glands have been inadvertently devascularized during primary surgical treatment for MTC, it is suggested that the glands needing reimplantation be implanted in the nondominant forearm. This approach minimizes the need for further surgical intervention in the neck should hyperparathyroidism develop or recur.[1,20,63,64] For children, the risk/benefit ratio must be carefully weighed to avoid overtreatment and subsequent aparathyroidism.[65] It is important to confirm that the remnant or autotransplanted parathyroid tissue is functional.[1,44,66,67]
Medical therapy of hyperparathyroidism has gained popularity with the advent of calcimimetics, agents that sensitize the calcium-sensing receptors on the parathyroid glands to circulating calcium levels and thereby reduce circulating parathyroid hormone (PTH) levels. In a randomized, double-blind, placebo-controlled trial, cinacalcet hydrochloride was shown to induce sustained reduction in circulating calcium and PTH levels in patients with PHPT.[68] In patients who are high-risk surgical candidates, those with recurrent hyperparathyroidism, or those in whom life expectancy is limited, medical therapy may be a viable alternative to a surgical approach.[1] Consequences of long-term therapy with cinacalcet are unknown.
References
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015. [PUBMED Abstract]
- Voss RK, Feng L, Lee JE, et al.: Medullary Thyroid Carcinoma in MEN2A: ATA Moderate- or High-Risk RET Mutations Do Not Predict Disease Aggressiveness. J Clin Endocrinol Metab 102 (8): 2807-2813, 2017. [PUBMED Abstract]
- Elisei R, Romei C, Renzini G, et al.: The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 years experience at one single center. J Clin Endocrinol Metab 97 (2): 426-35, 2012. [PUBMED Abstract]
- Rich TA, Feng L, Busaidy N, et al.: Prevalence by age and predictors of medullary thyroid cancer in patients with lower risk germline RET proto-oncogene mutations. Thyroid 24 (7): 1096-106, 2014. [PUBMED Abstract]
- Qi XP, Zhao JQ, Du ZF, et al.: Prophylactic thyroidectomy for MEN 2-related medullary thyroid carcinoma based on predictive testing for RET proto-oncogene mutation and basal serum calcitonin in China. Eur J Surg Oncol 39 (9): 1007-12, 2013. [PUBMED Abstract]
- Machens A, Lorenz K, Dralle H: Individualization of lymph node dissection in RET (rearranged during transfection) carriers at risk for medullary thyroid cancer: value of pretherapeutic calcitonin levels. Ann Surg 250 (2): 305-10, 2009. [PUBMED Abstract]
- Castagna MG, Fugazzola L, Maino F, et al.: Reference range of serum calcitonin in pediatric population. J Clin Endocrinol Metab 100 (5): 1780-4, 2015. [PUBMED Abstract]
- Kuhlen M, Frühwald MC, Dunstheimer DPA, et al.: Revisiting the genotype-phenotype correlation in children with medullary thyroid carcinoma: A report from the GPOH-MET registry. Pediatr Blood Cancer 67 (4): e28171, 2020. [PUBMED Abstract]
- Schreinemakers JM, Vriens MR, Valk GD, et al.: Factors predicting outcome of total thyroidectomy in young patients with multiple endocrine neoplasia type 2: a nationwide long-term follow-up study. World J Surg 34 (4): 852-60, 2010. [PUBMED Abstract]
- Torresan F, Censi S, Pennelli G, et al.: Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients. Cancers (Basel) 14 (24): , 2022. [PUBMED Abstract]
- Skinner MA, Moley JA, Dilley WG, et al.: Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med 353 (11): 1105-13, 2005. [PUBMED Abstract]
- Szinnai G, Meier C, Komminoth P, et al.: Review of multiple endocrine neoplasia type 2A in children: therapeutic results of early thyroidectomy and prognostic value of codon analysis. Pediatrics 111 (2): E132-9, 2003. [PUBMED Abstract]
- Brauckhoff M, Machens A, Lorenz K, et al.: Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann Surg 259 (4): 800-6, 2014. [PUBMED Abstract]
- Ordóñez J, Pérez-Egido L, García-Casillas MA, et al.: Management and results of thyroidectomies in pediatric patients with MEN 2 syndrome. J Pediatr Surg 56 (11): 2058-2061, 2021. [PUBMED Abstract]
- Franc S, Niccoli-Sire P, Cohen R, et al.: Complete surgical lymph node resection does not prevent authentic recurrences of medullary thyroid carcinoma. Clin Endocrinol (Oxf) 55 (3): 403-9, 2001. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Prophylactic neck surgery for second-generation multiple endocrine neoplasia type 2B. Eur J Surg Oncol 47 (4): 924-927, 2021. [PUBMED Abstract]
- Zenaty D, Aigrain Y, Peuchmaur M, et al.: Medullary thyroid carcinoma identified within the first year of life in children with hereditary multiple endocrine neoplasia type 2A (codon 634) and 2B. Eur J Endocrinol 160 (5): 807-13, 2009. [PUBMED Abstract]
- Hansen HS, Torring H, Godballe C, et al.: Is thyroidectomy necessary in RET mutations carriers of the familial medullary thyroid carcinoma syndrome? Cancer 89 (4): 863-7, 2000. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Lymph node metastasis in hereditary medullary thyroid cancer is independent of the underlying RET germline mutation. Eur J Surg Oncol 47 (4): 920-923, 2021. [PUBMED Abstract]
- Moley JF, Skinner M, Gillanders WE, et al.: Management of the Parathyroid Glands During Preventive Thyroidectomy in Patients With Multiple Endocrine Neoplasia Type 2. Ann Surg 262 (4): 641-6, 2015. [PUBMED Abstract]
- Machens A, Schneyer U, Holzhausen HJ, et al.: Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab 90 (4): 2029-34, 2005. [PUBMED Abstract]
- Spanheimer PM, Ganly I, Chou J, et al.: Long-Term Oncologic Outcomes After Curative Resection of Familial Medullary Thyroid Carcinoma. Ann Surg Oncol 26 (13): 4423-4429, 2019. [PUBMED Abstract]
- Razvi S, Hostalek U: Therapeutic challenges in the application of serum thyroid stimulating hormone testing in the management of patients with hypothyroidism on replacement thyroid hormone therapy: a review. Curr Med Res Opin 35 (7): 1215-1220, 2019. [PUBMED Abstract]
- Sawin CT, Geller A, Hershman JM, et al.: The aging thyroid. The use of thyroid hormone in older persons. JAMA 261 (18): 2653-5, 1989. [PUBMED Abstract]
- Baloch Z, Carayon P, Conte-Devolx B, et al.: Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13 (1): 3-126, 2003. [PUBMED Abstract]
- Seib CD, Harari A, Conte FA, et al.: Utility of serum thyroglobulin measurements after prophylactic thyroidectomy in patients with hereditary medullary thyroid cancer. Surgery 156 (2): 394-8, 2014. [PUBMED Abstract]
- Moley JF, Debenedetti MK, Dilley WG, et al.: Surgical management of patients with persistent or recurrent medullary thyroid cancer. J Intern Med 243 (6): 521-6, 1998. [PUBMED Abstract]
- Samaan NA, Schultz PN, Hickey RC: Medullary thyroid carcinoma: prognosis of familial versus nonfamilial disease and the role of radiotherapy. Horm Metab Res Suppl 21: 21-5, 1989. [PUBMED Abstract]
- Scherübl H, Raue F, Ziegler R: Combination chemotherapy of advanced medullary and differentiated thyroid cancer. Phase II study. J Cancer Res Clin Oncol 116 (1): 21-3, 1990. [PUBMED Abstract]
- Subbiah V, Hu MI, Wirth LJ, et al.: Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 9 (8): 491-501, 2021. [PUBMED Abstract]
- Wirth LJ, Sherman E, Robinson B, et al.: Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 383 (9): 825-835, 2020. [PUBMED Abstract]
- Wells SA, Robinson BG, Gagel RF, et al.: Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30 (2): 134-41, 2012. [PUBMED Abstract]
- Fox E, Widemann BC, Chuk MK, et al.: Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res 19 (15): 4239-48, 2013. [PUBMED Abstract]
- Kraft IL, Akshintala S, Zhu Y, et al.: Outcomes of Children and Adolescents with Advanced Hereditary Medullary Thyroid Carcinoma Treated with Vandetanib. Clin Cancer Res 24 (4): 753-765, 2018. [PUBMED Abstract]
- Elisei R, Schlumberger MJ, Müller SP, et al.: Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31 (29): 3639-46, 2013. [PUBMED Abstract]
- Schlumberger M, Elisei R, Müller S, et al.: Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol 28 (11): 2813-2819, 2017. [PUBMED Abstract]
- Sherman SI, Clary DO, Elisei R, et al.: Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 122 (24): 3856-3864, 2016. [PUBMED Abstract]
- Carlomagno F, Guida T, Anaganti S, et al.: Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 23 (36): 6056-63, 2004. [PUBMED Abstract]
- Subbiah V, Velcheti V, Tuch BB, et al.: Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 29 (8): 1869-1876, 2018. [PUBMED Abstract]
- Drilon A, Hu ZI, Lai GGY, et al.: Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15 (3): 151-167, 2018. [PUBMED Abstract]
- Redaelli S, Plaza-Menacho I, Mologni L: Novel targeted therapeutics for MEN2. Endocr Relat Cancer 25 (2): T53-T68, 2018. [PUBMED Abstract]
- Mulligan LM: Progress and potential impact of RET kinase targeting in cancer. Expert Rev Proteomics 13 (7): 631-3, 2016. [PUBMED Abstract]
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PUBMED Abstract]
- Kloos RT, Eng C, Evans DB, et al.: Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19 (6): 565-612, 2009. [PUBMED Abstract]
- Lenders JW, Duh QY, Eisenhofer G, et al.: Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99 (6): 1915-42, 2014. [PUBMED Abstract]
- Castinetti F, Qi XP, Walz MK, et al.: Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an international retrospective population-based study. Lancet Oncol 15 (6): 648-55, 2014. [PUBMED Abstract]
- Thosani S, Ayala-Ramirez M, Palmer L, et al.: The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab 98 (11): E1813-9, 2013. [PUBMED Abstract]
- Machens A, Lorenz K, Weber F, et al.: Recurrent ipsilateral pheochromocytoma in carriers of RET p.Cys634 missense mutations. Endocrine 77 (1): 160-167, 2022. [PUBMED Abstract]
- Lairmore TC, Ball DW, Baylin SB, et al.: Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndromes. Ann Surg 217 (6): 595-601; discussion 601-3, 1993. [PUBMED Abstract]
- Inabnet WB, Caragliano P, Pertsemlidis D: Pheochromocytoma: inherited associations, bilaterality, and cortex preservation. Surgery 128 (6): 1007-11;discussion 1011-2, 2000. [PUBMED Abstract]
- Scholten A, Valk GD, Ulfman D, et al.: Unilateral subtotal adrenalectomy for pheochromocytoma in multiple endocrine neoplasia type 2 patients: a feasible surgical strategy. Ann Surg 254 (6): 1022-7, 2011. [PUBMED Abstract]
- Grubbs EG, Rich TA, Ng C, et al.: Long-term outcomes of surgical treatment for hereditary pheochromocytoma. J Am Coll Surg 216 (2): 280-9, 2013. [PUBMED Abstract]
- Walz MK, Alesina PF, Wenger FA, et al.: Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery 140 (6): 943-8; discussion 948-50, 2006. [PUBMED Abstract]
- Walz MK, Alesina PF, Wenger FA, et al.: Laparoscopic and retroperitoneoscopic treatment of pheochromocytomas and retroperitoneal paragangliomas: results of 161 tumors in 126 patients. World J Surg 30 (5): 899-908, 2006. [PUBMED Abstract]
- Perrier ND, Kennamer DL, Bao R, et al.: Posterior retroperitoneoscopic adrenalectomy: preferred technique for removal of benign tumors and isolated metastases. Ann Surg 248 (4): 666-74, 2008. [PUBMED Abstract]
- Behrman SW, Bahr MH, Dickson PV, et al.: The microbiology of secondary and postoperative pancreatic infections: implications for antimicrobial management. Arch Surg 146 (5): 613-9, 2011. [PUBMED Abstract]
- Evans DB, Perrier ND: On "Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients". Surgery 140 (6): 951-2, 2006. [PUBMED Abstract]
- Dickson PV, Jimenez C, Chisholm GB, et al.: Posterior retroperitoneoscopic adrenalectomy: a contemporary American experience. J Am Coll Surg 212 (4): 659-65; discussion 665-7, 2011. [PUBMED Abstract]
- Cabalag MS, Mann GB, Gorelik A, et al.: Posterior retroperitoneoscopic adrenalectomy: outcomes and lessons learned from initial 50 cases. ANZ J Surg 85 (6): 478-82, 2015. [PUBMED Abstract]
- Prete A, Paragliola RM, Salvatori R, et al.: MANAGEMENT OF CATECHOLAMINE-SECRETING TUMORS IN PREGNANCY: A REVIEW. Endocr Pract 22 (3): 357-70, 2016. [PUBMED Abstract]
- Meijs AC, Snel M, Corssmit EPM: Pheochromocytoma/paraganglioma crisis: case series from a tertiary referral center for pheochromocytomas and paragangliomas. Hormones (Athens) 20 (2): 395-403, 2021. [PUBMED Abstract]
- Kraimps JL, Denizot A, Carnaille B, et al.: Primary hyperparathyroidism in multiple endocrine neoplasia type IIa: retrospective French multicentric study. Groupe d'Etude des Tumeurs á Calcitonine (GETC, French Calcitonin Tumors Study Group), French Association of Endocrine Surgeons. World J Surg 20 (7): 808-12; discussion 812-3, 1996. [PUBMED Abstract]
- Norton JA, Brennan MF, Wells SA Jr: Surgical Management of Hyperparathyroidism. In: Bilezikian JP, Marcus R, Levine MA: The Parathyroids: Basic and Clinical Concepts. Raven Press, 1994, pp 531-551.
- Scholten A, Schreinemakers JM, Pieterman CR, et al.: Evolution of surgical treatment of primary hyperparathyroidism in patients with multiple endocrine neoplasia type 2A. Endocr Pract 17 (1): 7-15, 2011 Jan-Feb. [PUBMED Abstract]
- Machens A, Dralle H: Advances in risk-oriented surgery for multiple endocrine neoplasia type 2. Endocr Relat Cancer 25 (2): T41-T52, 2018. [PUBMED Abstract]
- Khan MI, Waguespack SG, Hu MI: Medical management of postsurgical hypoparathyroidism. Endocr Pract 17 (Suppl 1): 18-25, 2011 Mar-Apr. [PUBMED Abstract]
- Stålberg P, Carling T: Familial parathyroid tumors: diagnosis and management. World J Surg 33 (11): 2234-43, 2009. [PUBMED Abstract]
- Peacock M, Bilezikian JP, Klassen PS, et al.: Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 90 (1): 135-41, 2005. [PUBMED Abstract]
Familial and Psychosocial Implications in Multiple Endocrine Neoplasia Type 2 (MEN2)
Attitudes Toward Preimplantation Genetic Testing
One study explored the attitudes of individuals with multiple endocrine neoplasia type 1 (MEN1) and multiple endocrine neoplasia type 2 (MEN2) toward preimplantation genetic testing (PGT).[1] Ninety-one clinic-based patients from a single U.S. institution who had MEN1 and an MEN1 pathogenic variant or MEN2 and a RET pathogenic variant were surveyed. The study found that 30% (10 of 33) of those with MEN1 and 37% (21 of 57) of those with MEN2 were aware of PGT; 82% (27 of 33) of those with MEN1 and 61% (34 of 56) of those with MEN2 thought PGT should be offered; and 61% (19 of 31) of those with MEN1 and 43% (23 of 54) of those with MEN2 would consider PGT.
Psychosocial Issues
The psychosocial impact of genetic testing for pathogenic variants in RET has not been extensively studied. Published studies have had limitations such as small sample size and heterogeneous populations, so the clinical relevance of these findings should be interpreted with caution. Identification as the carrier of a pathogenic variant may affect self-esteem, family relationships, and quality of life.[2] In addition, misconceptions about genetic disease may result in familial blame and guilt.[3,4] Several review articles outline both the medical and psychological issues, especially those related to the testing of children.[5-8] The medical value of early screening and risk-reducing treatment are contrasted with the loss of decision-making autonomy for the individual. Lack of agreement between parents about the value and timing of genetic testing and surgery may spur the development of emotional problems within the family.
One study examined levels of psychological distress in the interval between submitting a blood sample and receiving genetic test results. Individuals who experienced the highest level of distress were younger than 25 years, single, and had a history of responding to stressful situations with anxiety.[9] Pathogenic variant–positive parents whose children received negative test results did not seem to be reassured, questioned the reliability of the DNA test, and were eager to continue screening of their noncarrier children.[10]
A small qualitative study (N = 21) evaluated how patients with multiple endocrine neoplasia type 2A and family members conceptualized participation in lifelong high-risk surveillance.[11] Ongoing surveillance was viewed as a reminder of a health threat. Acceptance and incorporation of lifelong surveillance into routine health care was essential for coping with the implications of this condition. Concern about genetic predisposition to cancer was peripheral to concerns about surveillance. Supportive interventions, such as Internet discussion forums, can serve as an ongoing means of addressing informational and support needs of patients with medullary thyroid cancer undergoing lifelong surveillance.[12]
References
- Rich TA, Liu M, Etzel CJ, et al.: Comparison of attitudes regarding preimplantation genetic diagnosis among patients with hereditary cancer syndromes. Fam Cancer 13 (2): 291-9, 2014. [PUBMED Abstract]
- Freyer G, Ligneau B, Schlumberger M, et al.: Quality of life in patients at risk of medullary thyroid carcinoma and followed by a comprehensive medical network: trends for future evaluations. Ann Oncol 12 (10): 1461-5, 2001. [PUBMED Abstract]
- Freyer G, Dazord A, Schlumberger M, et al.: Psychosocial impact of genetic testing in familial medullary-thyroid carcinoma: a multicentric pilot-evaluation. Ann Oncol 10 (1): 87-95, 1999. [PUBMED Abstract]
- Grosfeld FJ, Lips CJ, Ten Kroode HF, et al.: Psychosocial consequences of DNA analysis for MEN type 2. Oncology (Huntingt) 10 (2): 141-6; discussion 146, 152, 157, 1996. [PUBMED Abstract]
- Johnston LB, Chew SL, Trainer PJ, et al.: Screening children at risk of developing inherited endocrine neoplasia syndromes. Clin Endocrinol (Oxf) 52 (2): 127-36, 2000. [PUBMED Abstract]
- MacDonald DJ, Lessick M: Hereditary cancers in children and ethical and psychosocial implications. J Pediatr Nurs 15 (4): 217-25, 2000. [PUBMED Abstract]
- Grosfeld FJ, Lips CJ, Beemer FA, et al.: Psychological risks of genetically testing children for a hereditary cancer syndrome. Patient Educ Couns 32 (1-2): 63-7, 1997 Sep-Oct. [PUBMED Abstract]
- Giarelli E: Multiple endocrine neoplasia type 2a (MEN2a): a call for psycho-social research. Psychooncology 11 (1): 59-73, 2002 Jan-Feb. [PUBMED Abstract]
- Grosfeld FJ, Lips CJ, Beemer FA, et al.: Distress in MEN 2 family members and partners prior to DNA test disclosure. Multiple endocrine neoplasia type 2. Am J Med Genet 91 (1): 1-7, 2000. [PUBMED Abstract]
- Grosfeld FJ, Beemer FA, Lips CJ, et al.: Parents' responses to disclosure of genetic test results of their children. Am J Med Genet 94 (4): 316-23, 2000. [PUBMED Abstract]
- Giarelli E: Bringing threat to the fore: participating in lifelong surveillance for genetic risk of cancer. Oncol Nurs Forum 30 (6): 945-55, 2003 Nov-Dec. [PUBMED Abstract]
- Schultz PN: Providing information to patients with a rare cancer: using Internet discussion forums to address the needs of patients with medullary thyroid carcinoma. Clin J Oncol Nurs 6 (4): 219-22, 2002 Jul-Aug. [PUBMED Abstract]
Latest Updates to This Summary (02/14/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Cancer Genetics Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about Multiple endocrine neoplasia type 2 (MEN2). It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Cancer Genetics Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Multiple Endocrine Neoplasia Type 2 (MEN2) are:
- Erica Blouch, MS, CGC (Massachusetts General Hospital Cancer Center)
- Kathleen A. Calzone, PhD, RN, AGN-BC, FAAN (National Cancer Institute)
- Suzanne C. O'Neill, PhD (Georgetown University)
- Nancy D. Perrier, MD, FACS (University of Texas, M.D. Anderson Cancer Center)
- John M. Quillin, PhD, MPH, MS (Virginia Commonwealth University)
- Charite Ricker, MS, CGC (University of Southern California)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Cancer Genetics Editorial Board. PDQ Multiple Endocrine Neoplasia Type 2 (MEN2). Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/publications/pdq/information-summaries/genetics/men2-hp-pdq. Accessed <MM/DD/YYYY>.
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.