Thymoma and Thymic Carcinoma Treatment (PDQ®)–Patient Version
General Information About Thymoma and Thymic Carcinoma
Key Points
- Thymoma and thymic carcinoma are diseases in which malignant (cancer) cells form in the thymus.
- Thymoma is linked with myasthenia gravis and other autoimmune paraneoplastic diseases.
- Signs and symptoms of thymoma and thymic carcinoma include a cough and chest pain.
- Tests that examine the thymus are used to help diagnose and stage thymoma and thymic carcinoma.
- Certain factors affect prognosis (chance of recovery) and treatment options.
Thymoma and thymic carcinoma are diseases in which malignant (cancer) cells form in the thymus.
Thymoma and thymic carcinoma, also called thymic epithelial tumors (TETs), are two types of rare cancers that can form in the cells that cover the outside surface of the thymus. The thymus is a small organ that lies in the upper chest above the heart and under the breastbone. It is part of the lymph system and makes white blood cells, called lymphocytes, that help fight infection. These cancers usually form between the lungs in the front part of the chest and are sometimes found during a chest x-ray that is done for another reason.
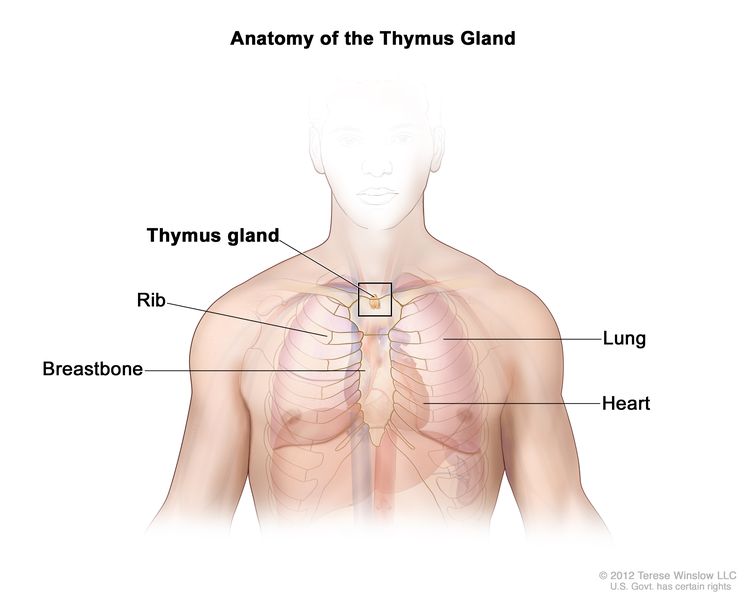
Even though thymoma and thymic carcinoma form in the same type of cell, they act differently:
- Thymoma. The cancer cells look a lot like the normal cells of the thymus, grow slowly, and rarely spread beyond the thymus.
- Thymic carcinoma. The cancer cells do not look like the normal cells of the thymus, grow more quickly, and are more likely to spread to other parts of the body. About one in every five TETs is a thymic carcinoma. Thymic carcinoma is more difficult to treat than thymoma.
Other types of tumors, such as lymphoma or germ cell tumors, may form in the thymus, but they are not considered to be thymoma or thymic carcinoma.
Thymoma is linked with myasthenia gravis and other autoimmune paraneoplastic diseases.
Autoimmune paraneoplastic diseases are often linked with thymoma. Autoimmune paraneoplastic diseases may occur in patients with cancer but are not caused directly by cancer. Autoimmune paraneoplastic diseases are marked by signs and symptoms that develop when the body's immune system attacks not only cancer cells but also normal cells. Autoimmune paraneoplastic diseases linked with thymoma include:
- Myasthenia gravis (the most common autoimmune paraneoplastic disease linked with thymoma).
- Thymoma-associated hypogammaglobulinemia (Good syndrome).
- Thymoma-associated autoimmune pure red cell aplasia.
Other autoimmune paraneoplastic diseases may be linked with TETs and can involve any organ.
Signs and symptoms of thymoma and thymic carcinoma include a cough and chest pain.
Most patients do not have signs or symptoms when first diagnosed with thymoma or thymic carcinoma. Check with your doctor if you have any of the following:
- A cough that doesn't go away.
- Shortness of breath.
- Chest pain.
- A hoarse voice.
- Swelling in the face, neck, upper body, or arms.
Tests that examine the thymus are used to help diagnose and stage thymoma and thymic carcinoma.
The following tests and procedures may be used:
- Physical exam and health history: An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
- Chest x-ray: An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, such as the chest, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- PET scan (positron emission tomography scan): A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
- MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body, such as the chest. This procedure is also called nuclear magnetic resonance imaging (NMRI).
- Biopsy: The removal of cells or tissues using a needle so they can be viewed under a microscope by a pathologist to check for signs of cancer.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis and treatment options depend on the following:
- Whether the cancer is thymoma or thymic carcinoma.
- Whether the cancer has spread to nearby areas or other parts of the body.
- Whether the tumor can be removed completely by surgery.
- Whether the cancer has just been diagnosed or has recurred (come back).
Stages of Thymoma and Thymic Carcinoma
Key Points
- After thymoma or thymic carcinoma has been diagnosed, tests are done to find out if cancer cells have spread to nearby areas or to other parts of the body.
- There are three ways that cancer spreads in the body.
- Cancer may spread from where it began to other parts of the body.
- The following stages are used for thymoma:
- Stage I
- Stage II
- Stage III
- Stage IV
- Thymic carcinomas have usually spread to other parts of the body when diagnosed.
- Thymic carcinoma is more likely to recur than thymoma.
After thymoma or thymic carcinoma has been diagnosed, tests are done to find out if cancer cells have spread to nearby areas or to other parts of the body.
The process used to find out if thymoma or thymic carcinoma has spread from the thymus to nearby areas or other parts of the body is called staging. Thymoma and thymic carcinoma may spread to the lungs, chest wall, major vessels, esophagus, or the lining around the lungs and heart. The results of tests and procedures done to diagnose thymoma or thymic carcinoma are used to help make decisions about treatment.
There are three ways that cancer spreads in the body.
Cancer can spread through tissue, the lymph system, and the blood:
- Tissue. The cancer spreads from where it began by growing into nearby areas.
- Lymph system. The cancer spreads from where it began by getting into the lymph system. The cancer travels through the lymph vessels to other parts of the body.
- Blood. The cancer spreads from where it began by getting into the blood. The cancer travels through the blood vessels to other parts of the body.
Cancer may spread from where it began to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood.
- Lymph system. The cancer gets into the lymph system, travels through the lymph vessels, and forms a tumor (metastatic tumor) in another part of the body.
- Blood. The cancer gets into the blood, travels through the blood vessels, and forms a tumor (metastatic tumor) in another part of the body.
The metastatic tumor is the same type of cancer as the primary tumor. For example, if thymic carcinoma spreads to the bone, the cancer cells in the bone are actually thymic carcinoma cells. The disease is metastatic thymic carcinoma, not bone cancer.
The following stages are used for thymoma:
Stage I
In stage I, cancer is found only within the thymus. All cancer cells are inside the capsule (sac) that surrounds the thymus.
Stage II
In stage II, cancer has spread through the capsule and into the fat around the thymus or into the lining of the chest cavity.
Stage III
In stage III, cancer has spread to nearby organs in the chest, including the lung, the sac around the heart, or large blood vessels that carry blood to the heart.
Stage IV
Stage IV is divided into stage IVA and stage IVB, depending on where the cancer has spread.
- In stage IVA, cancer has spread widely around the lungs or heart.
- In stage IVB, cancer has spread to the blood or lymph system.
Thymic carcinomas have usually spread to other parts of the body when diagnosed.
The staging system used for thymomas is sometimes used for thymic carcinomas.
Thymic carcinoma is more likely to recur than thymoma.
Recurrent thymoma and thymic carcinoma are cancers that have recurred (come back) after treatment. The cancer may come back in the thymus or in other parts of the body. Thymic carcinoma is more likely to recur than thymoma.
- Thymomas may recur a long time after treatment is completed. There is also an increased risk of having another type of cancer after having a thymoma. For these reasons, lifelong follow-up is needed.
- Thymic carcinomas often recur.
Treatment Option Overview
Key Points
- There are different types of treatment for patients with thymoma and thymic carcinoma.
- The following types of treatment are used:
- Surgery
- Radiation therapy
- Chemotherapy
- Hormone therapy
- Targeted therapy
- New types of treatment are being tested in clinical trials.
- Immunotherapy
- Treatment for thymoma and thymic carcinoma may cause side effects.
- Patients may want to think about taking part in a clinical trial.
- Patients can enter clinical trials before, during, or after starting their cancer treatment.
- Follow-up tests may be needed.
There are different types of treatment for patients with thymoma and thymic carcinoma.
Different types of treatments are available for patients with thymoma and thymic carcinoma. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
The following types of treatment are used:
Surgery
Surgery to remove the tumor is the most common treatment of thymoma.
After the doctor removes all the cancer that can be seen at the time of the surgery, some patients may be given radiation therapy after surgery to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called adjuvant therapy.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy).
Chemotherapy may be used to shrink the tumor before surgery or radiation therapy. This is called neoadjuvant chemotherapy.
Hormone therapy
Hormone therapy is a cancer treatment that removes hormones or blocks their action and stops cancer cells from growing. Hormones are substances made by glands in the body and flow through the bloodstream. Some hormones can cause certain cancers to grow. If tests show that the cancer cells have places where hormones can attach (receptors), drugs, surgery, or radiation therapy is used to reduce the production of hormones or block them from working. Hormone therapy using octreotide with or without prednisone may be used to treat thymoma or thymic carcinoma.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR) inhibitors are types of targeted therapies used in the treatment of thymoma and thymic carcinoma.
- TKIs: This treatment blocks signals needed for tumors to grow. Sunitinib and lenvatinib are TKIs that may be used to treat recurrent thymoma or recurrent thymic carcinoma.
- mTOR inhibitors: This treatment blocks a protein called mTOR, which may keep cancer cells from growing and prevent the growth of new blood vessels that tumors need to grow. Everolimus is an mTOR inhibitor that may be used to treat recurrent thymoma or recurrent thymic carcinoma.
New types of treatment are being tested in clinical trials.
This summary section describes treatments that are being studied in clinical trials. It may not mention every new treatment being studied. Information about clinical trials is available from the NCI website.
Immunotherapy
Immunotherapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This cancer treatment is a type of biologic therapy.
- Immune checkpoint inhibitor therapy: PD-1 is a protein on the surface of T cells that helps keep the body’s immune responses in check. PD-L1 is a protein found on some types of cancer cells. When PD-1 attaches to PD-L1, it stops the T cell from killing the cancer cell. PD-1 and PD-L1 inhibitors keep PD-1 and PD-L1 proteins from attaching to each other. This allows the T cells to kill cancer cells. Pembrolizumab is a type of PD-1 inhibitor that is being studied in the treatment of recurrent thymoma and thymic carcinoma.
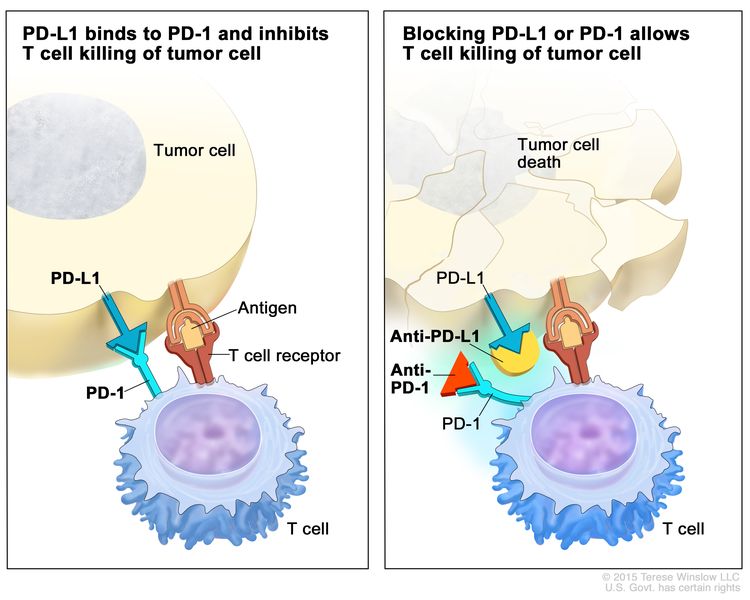
Treatment for thymoma and thymic carcinoma may cause side effects.
For information about side effects caused by treatment for cancer, visit our Side Effects page.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back).
Treatment of Stage I and Stage II Thymoma
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage I and stage II thymoma is surgery, which may be followed by radiation therapy.
Treatment of Stage III and Stage IV Thymoma
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage III and stage IV thymoma that may be completely removed by surgery includes the following:
- Surgery followed by radiation therapy.
- Neoadjuvant chemotherapy followed by surgery and radiation therapy.
Treatment of stage III and stage IV thymoma that cannot be completely removed by surgery includes the following:
- Chemotherapy.
- Chemotherapy followed by radiation therapy.
- Neoadjuvant chemotherapy followed by surgery (if operable) and radiation therapy.
Treatment of Thymic Carcinoma
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of thymic carcinoma that may be completely removed by surgery includes the following:
- Surgery followed by radiation therapy with or without chemotherapy.
Treatment of thymic carcinoma that cannot be completely removed by surgery includes the following:
- Chemotherapy.
- Chemotherapy with radiation therapy.
- Chemotherapy followed surgery, if the tumor may be completely removed, and radiation therapy.
Treatment of Recurrent Thymoma and Thymic Carcinoma
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of recurrent thymoma and thymic carcinoma may include the following:
- Chemotherapy.
- Hormone therapy (octreotide) with or without prednisone.
- Targeted therapy.
- Surgery.
- Radiation therapy.
- A clinical trial of immune checkpoint inhibitor therapy with pembrolizumab.
To Learn More About Thymoma and Thymic Carcinoma
For more information from the National Cancer Institute about thymoma and thymic carcinoma, see the following:
For general cancer information and other resources from the National Cancer Institute, visit:
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of adult thymoma and thymic carcinoma. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Thymoma and Thymic Carcinoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/thymoma/patient/thymoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389395]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.