Cancer Currents: An NCI Cancer Research Blog
A blog featuring news and research updates from the National Cancer Institute. Learn more about Cancer Currents.
-
Moving Beyond BMI: Low Muscle Mass May Affect Cancer Survival
Researchers compared the risk of death for women with breast cancer who had low skeletal muscle mass, or sarcopenia, at the time of their cancer diagnosis and women who had adequate muscle mass.
-
Immunotherapy Drugs Expand Treatment Options for Advanced Lung Cancer
Results from a large clinical trial show combining an immune checkpoint inhibitor with chemotherapy helped some patients with advanced lung cancer live longer than chemotherapy alone. How will this change the lung cancer treatment landscape?
-
Cancer Control in American Indian and Alaska Native Populations: A Conversation with Dr. Shobha Srinivasan
American Indian and Alaska Native populations are disproportionately affected by certain cancers. In this interview, Dr. Shobha Srinivasan discusses some of these disparities and programs funded by NCI that are helping to address them.
-
Take with Food: Study Tests Lowering Dose of Prostate Cancer Drug
In a small clinical trial, researchers compared the efficacy of a much lower dose of the cancer drug abiraterone (Zytiga) taken with a low-fat breakfast with a full dose taken on an empty stomach, as directed on the drug’s label.
-
NCI Expands Repository of Cancer Research Models: A Conversation with Drs. Doroshow and Evrard
NCI is expanding its Patient-Derived Models Repository (PDMR), which provides cancer research models made directly from human tumor tissue. In this Q&A, Drs. Yvonne Evrard and James Doroshow explain how the new models can help cancer researchers make more rapid progress.
-
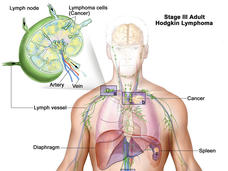
Brentuximab Approved for Initial Treatment of Advanced Hodgkin Lymphoma
The FDA has expanded the approved uses of brentuximab (Adcetris) in people with Hodgkin lymphoma. Under the new approval, brentuximab can be used in combination with three other chemotherapy drugs as an initial treatment in patients with advanced disease.
-
Advancing Patient Care Through Focused Innovation
NCI Director Dr. Norman Sharpless describes the focus areas of opportunity he has identified that, with enhanced attention from NCI, he believes can accelerate progress in cancer research and care.
-
Interactive App Improves Colorectal Cancer Screening Rates
Colorectal cancer screening reduces deaths from the disease, yet about one-third of Americans aren’t up to date with screening. Learn what happened when people waiting for routine checkups used an app that allowed them to order their own screening test.
-
Cancer Immunotherapy Drug Simultaneously Targets Two Proteins that Block Immune Response
Two independent groups of researchers have fused a TGF-beta receptor to a monoclonal antibody that targets a checkpoint protein. The result is a single hybrid molecule called a Y-trap that blocks two pathways used by tumors to evade the immune system.
-
NCI Launches New Resource for Specimens and Data from Cancer Clinical Trials
NCI has launched Navigator, a new resource for researchers interested in using specimens and clinical data collected from large cancer clinical trials.
-
Forging Military Partnerships to Empower the Cancer Research Enterprise
Partnerships and collaborations are an important component of NCI’s success. NCI Director Dr. Norman Sharpless describes three efforts made possible by a memorandum of agreement with three US military institutions: the APOLLO network, NAVIGATE, and BD-STEP.
-
Higher Risk of Heart Failure Seen in Some Cancers
Some people who have been treated for breast cancer or lymphoma have a higher risk of developing congestive heart failure than people who haven’t had cancer, results from a new study show.
-
Many Men with Penile Cancer Do Not Get Recommended Treatments, Study Finds
An analysis of records from a national cancer treatment database has found that many men with penile cancer that has not spread beyond nearby lymph nodes did not undergo lymph node biopsy or receive chemotherapy, as recommended by widely used professional guidelines.
-
Abemaciclib Approval Expands Initial Treatment Options for Advanced Breast Cancer
FDA has approved the CDK4/6 inhibitor abemaciclib (Verzenio) as a first-line treatment in some women with advanced or metastatic breast cancer. Under the approval, the drug must be used in combination with an aromatase inhibitor.
-
The Imperative of Addressing Cancer Drug Costs and Value
The President’s Cancer Panel has released its latest report, Promoting Value, Affordability, and Innovation in Cancer Drug Treatment. The report recommends six actions to maximize the value and affordability of cancer drug treatment.
-
Drug Combination Reduces Number of Colorectal Polyps in Patients with Hereditary Cancer Syndrome
People with FAP, an inherited condition that greatly increases their risk of gastrointestinal cancer, who took the drugs erlotinib (Tarceva) and sulindac (Aflodac) saw a substantial decrease in the number of precancerous lesions in the colon and rectum.
-
Drug May Help Prevent Resistance to Toxin-Based Leukemia Therapy
A new study has identified a possible strategy for improving the efficacy of a toxin-based cancer treatment, moxetumomab pasudotox, in some patients with acute lymphoblastic leukemia (ALL).
-
Targeted Therapy Larotrectinib Shows Promise in Early Trials, Regardless of Cancer Type
Initial results from a series of three small clinical trials of a targeted cancer therapy called larotrectinib suggest that it may be effective in patients—children and adults—with a wide variety of cancer types.
-
FDA Approves Apalutamide for Some Men with Prostate Cancer
In the trial that led to the approval, apalutamide (Erleada) delayed cancer metastasis for men with prostate cancer that is resistant to androgen deprivation therapy.
-
Testing an Interactive Approach to Promote Exercise in Young Cancer Survivors
An interactive website designed to promote physical activity among children and adolescents who have completed treatment for cancer may indeed help encourage them to get regular exercise, according to preliminary results from a pilot study.