Cancer Currents: An NCI Cancer Research Blog
A blog featuring news and research updates from the National Cancer Institute.
-
HPV Vaccination Linked to Decreased Oral HPV Infections
A study of more than 2,600 young adults found that the prevalence of oral infection with four HPV types, including two cancer-causing types, was 88% lower in those who reported receiving at least one dose of an HPV vaccine than in those not vaccinated.
-
Midostaurin Approved by FDA for Acute Myeloid Leukemia
The FDA has approved midostaurin for patients with newly diagnosed acute myeloid leukemia (AML) with mutations in the FLT3 gene. The approval also covers several rare conditions.
-
Study Identifies Genetic Mutations in Tumors From 10,000 Patients with Metastatic Cancer
Researchers at Memorial Sloan Kettering Cancer Center have reported the results of an initiative to characterize the genetic mutations in tumors from more than 10,000 patients with advanced cancer treated at the center.
-
FDA Approves Immunotherapy Drugs for Patients with Bladder Cancer
The FDA has approved four immunotherapy drugs—avelumab, atezolizumab, durvalumab, and pembrolizumab—for the treatment of patients with bladder cancer. All four drugs belong to a class of cancer therapies known as checkpoint inhibitors.
-
Intensive Pre-Stem Cell Transplant Regimen May be Best for Younger Patients with AML, MDS
Results from a large phase III clinical trial suggest that a highly intensive preparatory regimen should be used for younger patients with acute myeloid leukemia or myelodysplastic syndromes preparing to undergo an allogeneic stem cell transplant.
-
Regorafenib Becomes First FDA-Approved Drug for Liver Cancer in Nearly a Decade
FDA approved the kinase inhibitor regorafenib for some patients with hepatocellular carcinoma, the most common form of liver cancer.
-
FDA Grants Brigatinib Accelerated Approval for Metastatic Non-Small Cell Lung Cancer
On April 28, the FDA granted accelerated approval to the targeted therapy brigatinib (Alunbrig™) for patients with metastatic non-small cell lung cancer (NSCLC) and alterations in the ALK gene whose cancer has progressed during their initial therapy.
-
Counseling Improves Survivorship Plan Implementation for Low-Income Breast Cancer Survivors
In a randomized trial, low-income women who role-played talking with their doctor about their survivorship care plan in a counseling session reported receiving more of their recommended care than women who did not get counseling.
-
NCI ALMANAC: A New Tool for Research on Cancer Drug Combinations
NCI has released a new, easy-to-use resource called the NCI ALMANAC to help researchers identify potentially promising combinations of cancer drugs.
-
Collection of Patient-Reported Outcomes Feasible in Cancer Clinical Trials
Cancer patients, even those who are undergoing difficult treatments, are willing to devote time to completing thorough assessments of the side effects they encounter in clinical trials, a new study finds.
-
Nanoparticles Create Effective CAR T Cells in Living Mice
Researchers have developed a method to genetically engineer cancer-fighting immune cells in living animals using nanoparticles that carry DNA. The new study shows that the resulting immune cells, known as CAR T cells, eliminated leukemia in mice.
-
Patients Who Choose No Intervention for Small Thyroid Cancers Report Lack of Support
Patients who choose not to pursue immediate biopsy or treatment for small, asymptomatic thyroid cancers, or suspected cancers, can experience a lack of support from doctors and loved ones, a new study shows.
-
PARP Inhibitors May Be Effective in Brain, Other Cancers with IDH Mutations
Studies presented at the 2017 AACR annual meeting suggest that therapies which take advantage of the mutations in the IDH gene may be more effective than drugs that block it.
-
Making Greater Progress Against Cancer—Not Just a Hope but a Reality
Acting NCI Director Dr. Doug Lowy discusses what he calls encouraging news in the most recent Annual Report to the Nation and how NCI is helping to achieve further progress against cancer.
-
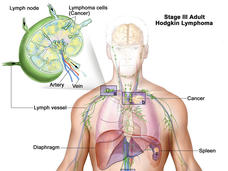
FDA Approves Pembrolizumab for Hodgkin Lymphoma
The FDA approved pembrolizumab for the treatment of some adult and pediatric patients with classical Hodgkin lymphoma.
-
Studies Identify Potential Treatment Strategies for Pediatric DIPG Brain Tumors
Two studies have identified proteins that drive growth of diffuse intrinsic pontine glioma (DIPG) tumor cells. Blocking these targets with investigational drugs slowed tumor growth in animal models.
-
FDA Approves Niraparib as Maintenance Therapy for Recurrent Ovarian Cancer
The FDA approved the PARP inhibitor niraparib for use as a maintenance therapy for some women with advanced ovarian cancer.
-
FDA Approves Ribociclib, Expands Palbociclib Approval for Metastatic Breast Cancer
The FDA has approved a new targeted therapy, ribociclib, and expanded its earlier approval of another targeted therapy, palbociclib, for some women with metastatic breast cancer.
-
Advancing the Potential and Promise of Total-Body PET Imaging
A total-body PET scanner under development is an ideal example of how NCI and NIH are supporting the development of new research and cancer care-related technologies.
-
Avelumab Becomes First Approved Treatment for Patients with Merkel Cell Carcinoma
The FDA has approved the first drug ever for the rare skin cancer, Merkel cell carcinoma, and updated data show an improved tumor response rate and that patients’ tumors continued to respond for at least a year.