Cancer Currents: An NCI Cancer Research Blog
A blog featuring news and research updates from the National Cancer Institute.
-
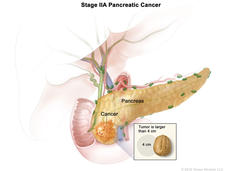
Altering Chemotherapy Improves Outcomes in Early-Stage Pancreatic Cancer
Results from two clinical trials are expected to improve the outlook for some people with early-stage pancreatic cancer. Altering the chemotherapy drugs used and the timing of treatment substantially improved survival.
-
New on NCI Websites for June 2018
NCI periodically provides updates on new websites and other online content of interest to the cancer community. See selected content that has been added as of June 2018.
-
Approval Expanded for Venetoclax in Chronic Lymphocytic Leukemia
FDA expanded the approval of venetoclax (Venclexta) for people with chronic lymphocytic leukemia (CLL) to include those whose cancer has progressed after previous treatment, regardless of whether their cancer cells have the deletion 17p gene alteration.
-
Do Frequent Follow-Up Tests Benefit Colorectal Cancer Survivors?
Two studies examined the impact of more frequent follow-up testing for cancer recurrence in colorectal cancer survivors. Learn whether the studies showed that frequent testing improved survival.
-
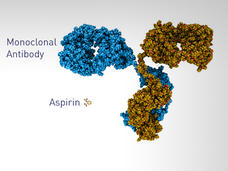
Biosimilars for Cancer Emerge as Patents on Widely Used Biological Drugs Expire
As the patents on some widely used drugs to treat cancer expire in the coming years, biosimilar drugs are being developed for the treatment of patients with cancer. Are biosimilars effective and will they expand treatment options for patients?
-
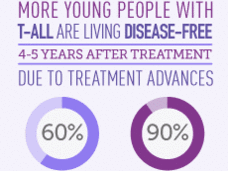
Trial Produces Practice-Changing Findings for Some Children, Young Adults with Leukemia
This NCI-funded Children’s Oncology Group trial tested the addition of nelarabine (Arranon) to standard treatment for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL).
-
OncoArray Links Dozens of DNA Variants to Risk for Common Cancers
Researchers with the NCI-supported GAME-ON initiative and OncoArray Network are publishing studies identifying dozens of new genetic variants associated with the risk for developing some of the most common cancers.
-
Maximizing the Prospects for Progress Against Cancer
NCI Director Dr. Norman Sharpless highlights some of the important research findings from the 2018 American Society of Clinical Oncology annual meeting and discusses the rapid pace of progress in cancer research.
-
Selumetinib Continues to Show Promise in Children with NF1
In a phase 2 clinical trial, the investigational drug selumetinib shrank tumors in some children with the genetic syndrome neurofibromatosis type 1 (NF1). The tumors, called neurofibromas, can cause pain, difficulty breathing or walking, and disfigurement.
-
Toxin-Based Drug Moxetumomab Pasudotox May Be New Option for Rare Leukemia
People diagnosed with hairy cell leukemia (HCL) may have an effective new treatment option, a type of drug called an immunotoxin. Read more about how this treatment, moxetumomab pasudotox, fared in a phase 3 clinical trial in patients with advanced HCL.
-
Easing Concerns about Giving Research Study Participants Their Genetic Test Results
Do cancer study participants want to receive their genetic test results? A recent study involving women with a history of breast cancer tested an approach for returning genetic research results and evaluated the impact those results had on the women.
-
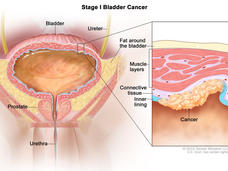
New Treatment Approach Could Help Prevent Recurrences of Some Bladder Cancers
Flushing the bladder with the chemotherapy drug gemcitabine after tumors have been removed surgically may reduce the risk of low-grade bladder cancer returning, according to the results of a large clinical trial.
-
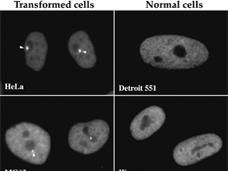
Experimental Cancer Drug Metarrestin Targets Metastatic Tumors
Researchers have struggled to develop therapies to treat tumors that have spread to other parts of the body. In a new study, researchers tested whether the experimental drug metarrestin can selectively shrink metastases in mouse models of aggressive pancreatic cancer.
-
Dabrafenib–Trametinib Combination Approved for Melanoma, Anaplastic Thyroid Cancer
FDA recently approved the targeted-drug combination to treat patients with advanced melanoma and a subset of patients with a rare and aggressive form of thyroid cancer whose tumors have a specific mutation in the BRAF gene.
-
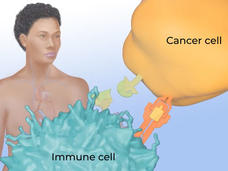
Can Immunotherapy Succeed in Glioblastoma?
Despite continued efforts to develop new therapies for glioblastoma, none have been able to improve how long patients live appreciably. Despite some setbacks, researchers are hopeful that immunotherapy might be able to succeed where other therapies have not.
-
Some Children with Wilms Tumor Can Receive Less Therapy, Study Suggests
Results from an NCI-sponsored clinical trial may point to an important change in how some children with advanced Wilms tumor, a form of kidney cancer, are treated.
-
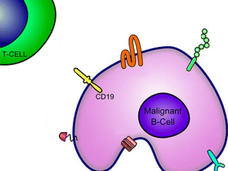
FDA Approves Second CAR T-Cell Therapy for Lymphoma
FDA has approved tisagenlecleucel (Kymriah) for certain kinds of non-Hodgkin lymphoma. Read about the trial that led to the approval and what the approval means for people with lymphoma.
-
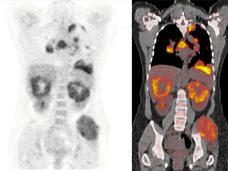
FDA Approves Nivolumab and Ipilimumab Combination for Advanced Kidney Cancer
FDA has approved the combination of two immunotherapy drugs, nivolumab (Opdivo) and ipilimumab (Yervoy), as an initial treatment for some patients with advanced kidney cancer. Learn how this approval will affect patient care.
-
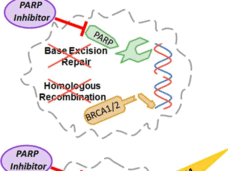
Rucaparib Approved as Maintenance Treatment for Some Recurrent Ovarian Cancers
FDA has expanded its approval of rucaparib (Rubraca) as a maintenance therapy for women with recurrent ovarian, fallopian tube, or primary peritoneal cancer whose tumors shrank after subsequent treatment with a platinum-based chemotherapy.
-
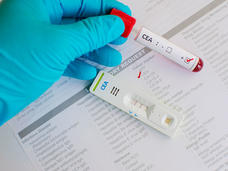
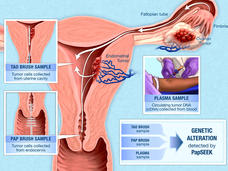
Study Shows Experimental Screening Test Can Detect Endometrial and Ovarian Cancers
Scientists have struggled to come up with a simple test to detect endometrial and ovarian cancers early, when they are most likely to respond to treatment. Can a liquid biopsy test called PapSEEK change that?