Cancer Currents: An NCI Cancer Research Blog
A blog featuring news and research updates from the National Cancer Institute.
-
Two Drugs Work Together to Block ‘Master Regulator’ of Breast, Other Cancers
Arsenic trioxide and retinoic acid work together to target the master regulator protein Pin1, a new study shows. In cancer cell lines and mice, the drug combination slowed the growth of triple-negative breast cancer tumors.
-
Study Provides Closer Look at Postmenopausal Bleeding and Endometrial Cancer
A new study has found that 90% of postmenopausal women diagnosed with endometrial cancer reported vaginal bleeding before their diagnosis. Approximately 9% of postmenopausal women who saw a doctor for bleeding, the study showed, later received an endometrial cancer diagnosis.
-
Enhancements to NCI’s SEER Program Creating New Research Opportunities
NCI’s SEER program is expanding in size and operating a series of innovative pilot studies. As Dr. Lynne Penberthy explains, these studies are setting the stage for the routine collection of more clinical and genomic data that will help researchers better understand cancer and its impact.
-
Ribociclib Approval Expanded for Some Women with Advanced Breast Cancer
FDA has expanded the approved uses of ribociclib (Kisqali) for women with advanced breast cancer, including new uses in pre- and postmenopausal women. It’s the first approval under a new FDA program to speed the review of cancer drugs.
-
Liquid Biopsy May Predict Risk of Breast Cancer Returning Years Later
Using a liquid biopsy to test for tumor cells circulating in blood, researchers found that, in women with breast cancer, the presence of these cells could identify women at risk of their cancer returning years later.
-
Targeted Drug Approved for Acute Myeloid Leukemia with IDH1 Gene Mutations
The FDA has approved ivosidenib (Tibsovo) for the treatment of adults with acute myeloid leukemia (AML) that has a specific mutation in a gene called IDH1. Ivosidenib becomes the first FDA-approved IDH1-targeted treatment.
-
Tailored Psychotherapy Eases Depression in People with Advanced Cancer
Just three to six sessions of a tailored psychotherapy approach called CALM helped to lessen symptoms of depression in people recently diagnosed with advanced cancer, results from a clinical trial show. The approach also may help prevent depression, researchers found.
-
High-Fat Diet or Diabetes Drug May Enhance Response to Targeted Cancer Drug
A study in mice may have identified a way to help overcome resistance to targeted cancer drugs known as PI3K inhibitors. The approach appears to work by reducing insulin levels in patients receiving these drugs.
-
Combination of Immunotherapy Drugs Approved for Metastatic Colorectal Cancer
The FDA has approved the combination of the immune checkpoint inhibitors ipilimumab (Yervoy) and nivolumab (Opdivo) for the treatment of patients with metastatic colorectal cancer whose tumor cells have defects that affect their ability to repair DNA.
-
New Immunotherapy Option Approved for Cervical Cancer, Rare Lymphoma
FDA has approved pembrolizumab (Keytruda) for some women with advanced cervical cancer and some patients with primary mediastinal large B-cell lymphoma (PMBCL), a rare type of non-Hodgkin lymphoma.
-
Aggressive Prostate Cancer Subtype More Common Than Expected
Researchers have found that men with advanced prostate cancer may be more likely than previously thought to develop a more aggressive form of the disease. The subtype, called t-SCNC, was linked with shorter survival than other subtypes.
-
Developing Biomarkers for Immunotherapy: A Conversation with Drs. Magdalena Thurin and Helen Chen
NCI is supporting a new research network to develop biomarkers for cancer immunotherapy. In this interview, NCI’s Dr. Helen Chen and Magdalena Thurin explain the networks’ structure and its goals.
-
FDA Alters Approved Use of Two Checkpoint Inhibitors for Bladder Cancer
FDA has changed the approved uses of the immunotherapy drugs pembrolizumab (Keytruda) and atezolizumab (Tecentriq) to treat the most common form of bladder cancer. The change is based on whether patients’ tumors have a specific biomarker.
-
Mouse Study Links Immune Cells to Diarrhea Caused by Chemotherapy
A study in mice sheds light onto how some chemotherapies cause diarrhea. The findings could be the basis for developing new treatments for patients with cancer who develop gastrointestinal side effects from chemotherapy.
-
The Opioid Epidemic and Cancer Pain Management: A Conversation with Dr. Judith Paice
Dr. Judith Paice, of the Cancer Pain Program at Northwestern University’s Feinberg School of Medicine, discusses the impacts of the opioid epidemic on cancer patients and how providers can address concerns about opioid misuse when managing cancer pain.
-
Sodium Thiosulfate Prevents Cisplatin-Induced Hearing Loss in Some Children
The drug sodium thiosulfate can protect the hearing of children with cancer undergoing treatment with the chemotherapy drug cisplatin, results from a new clinical trial show. The trial involved children with a form of liver cancer called hepatoblastoma.
-
Cancer Prevention Message Is Key for HPV Vaccination Discussions with Parents
Health care providers should emphasize cancer prevention when discussing HPV vaccination with the parents of preteens who are due to receive the vaccine, results from a new study show.
-
Can Age Affect Response to Immune Checkpoint Inhibitors?
A new study has linked age with how well patients with melanoma responded to treatment with immune checkpoint inhibitors. Experiments in mice suggested that the response pattern may be due to an age-related shift in the kinds of immune cells in tumors.
-
Dr. Alan S. Rabson, Long-Time NCI Deputy Director and Cancer Research Stalwart, Dies
Many in the cancer research and NIH community are mourning the loss of long-time NCI senior leader Alan Rabson, M.D., who passed away on July 4, at the age of 92. His career spanned 6 decades, including research on tumor virology and cancer pathology and senior NCI leadership roles.
-
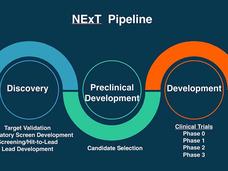
NExT: Advancing Promising Cancer Therapies from the Lab to Clinical Trials
The NCI Experimental Therapeutics (NExT) program works with researchers and top scientific experts to advance promising or novel cancer therapies from the earliest stages of research to human clinical trials.