Cancer Currents: An NCI Cancer Research Blog
A blog featuring news and research updates from the National Cancer Institute.
-
How Does Ovarian Cancer Form? A New Study Points to MicroRNA
A microRNA—a molecule made by cells to turn genes on and off—called miR-181a may help high-grade serous ovarian cancer form, a study has found. The scientists think the microRNA could potentially help doctors detect ovarian cancer earlier.
-
How CRISPR Is Changing Cancer Research and Treatment
The gene-editing tool CRISPR is changing the way scientists study cancer, and may change how cancer is treated. This in-depth blog post describes how this revolutionary technology is being used to better understand cancer and create new treatments.
-
Liquid Biopsy Detects Brain Cancer and Early-Stage Kidney Cancer
Results from two studies show that a liquid biopsy that analyzes DNA in blood accurately detected kidney cancer at early and more advanced stages and identified and classified different types of brain tumors.
-
Rediscovered Drugs Hit Leukemia from Two Different Angles
Two rediscovered drugs, bisantrene and brequinar, slowed the growth of acute myeloid leukemia in studies of mice. The drugs blocked the activity of a protein called FTO, killing cancer stem cells and helping the immune system attack the cancer.
-
Ovarian Cancer Studies Aim to Reduce Racial Disparities, Improve Outcomes
Three recently launched NCI-supported studies could help researchers better understand the causes of racial/ethnic disparities in ovarian cancer. The ultimate goal is to eliminate disparities and improve survival for all women with the disease.
-
CCDI Activities Enhance NCI’s Childhood and AYA Cancer Research
Since launching the Childhood Cancer Data Initiative, NCI has undertaken a range of research activities to support this important effort. In this Cancer Currents post, NCI Director Dr. Norman Sharpless provides an update on these efforts.
-
Study Links Mental Health Treatment to Improved Cancer Survival
In a study of more than 50,000 veterans with lung cancer, those with mental illness who received mental health treatment—including for substance use—lived substantially longer than those who didn’t participate in such programs.
-
A New FDA Approval Furthers the Role of Genomics in Cancer Care
FDA’s approval of pembrolizumab (Keytruda) to treat people whose cancer is tumor mutational burden-high highlights the importance of genomic testing to guide treatment, including for children with cancer, according to NCI Director Dr. Ned Sharpless.
-
New Drug Regimen Cures More Children with Aggressive B-Cell Lymphoma
For children with aggressive Burkitt lymphoma and other B-cell non-Hodgkin lymphomas, adding rituximab (Rituxan, Truxima) to chemotherapy substantially increases the likelihood of the child being cured, results from a large clinical trial show.
-
Responding to Coronavirus, Cancer Researchers Reimagine Clinical Trials
In response to the COVID-19 pandemic, cancer researchers are making changes to clinical trials to ensure patient safety and protect the integrity of their work. Some changes, such as greater use of telemedicine, will likely continue into the future.
-
Study Clarifies Timing of Immunotherapy for Advanced Bladder Cancer
Results from a large study show that, for most people with advanced bladder cancer, starting immunotherapy with avelumab (Bavencio) shortly after initial treatment with chemotherapy is better than delaying treatment.
-
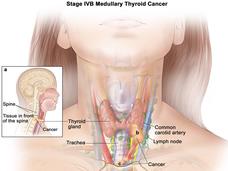
Selpercatinib Approved for Thyroid and Lung Cancers with RET Gene Alterations
FDA has granted accelerated approval for selpercatinib (Retevmo) to treat certain patients with thyroid cancer or non-small cell lung cancer whose tumors have RET gene alterations. The drug, which works by blocking the activity of RET proteins, was approved based on the results of the LIBRETTO-001 trial.
-
Olanzapine Reduces Nausea and Vomiting Caused by Advanced Cancer
Many advanced cancer patients suffer from chronic nausea and vomiting and there aren’t many good treatments available. But a small study suggests that the drug olanzapine (Zyprexa) may fill that gap.
-
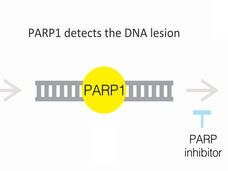
With Two FDA Approvals, Prostate Cancer Treatment Enters the PARP Era
FDA has approved olaparib (Lynparza) and rucaparib (Rubraca) to treat some men with metastatic prostate cancer. The PARP inhibitors are approved for men whose cancers have stopped responding to hormone treatment and have specific genetic alterations.
-
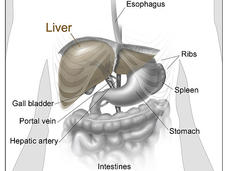
Atezolizumab Plus Bevacizumab Approved to Treat Liver Cancer
FDA has approved atezolizumab (Tecentriq) plus bevacizumab (Avastin) as an initial treatment for some people with advanced liver cancer. This is the first approval in 13 years for a treatment that is more effective than the current standard, sorafenib.
-
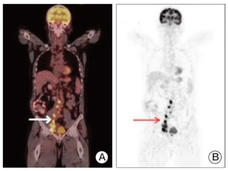
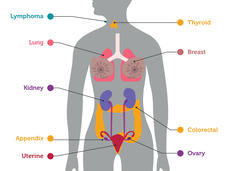
Study Examines Whether Blood Test Can Identify Early Cancers
A blood test combined with imaging tests detected tumors—some at an early stage—in women with no history of cancer or symptoms, a recent study showed. The test also mistakenly indicated some women had cancer when further testing showed they didn't.
-
More Evidence that Ruxolitinib Benefits Some Patients with Graft-Versus-Host Disease
Patients with acute graft-versus-host disease (GVHD) that does not respond to steroid therapy are more likely to respond to the drug ruxolitinib (Jakafi) than other available treatments, results from a large clinical trial show.
-
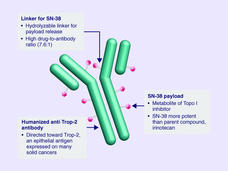
Sacituzumab Govitecan Approved for Metastatic Triple-Negative Breast Cancer
FDA has approved sacituzumab govitecan (Trodelvy) for the treatment of triple-negative breast cancer that has spread to other parts of the body. Under the approval, patients must have already undergone at least two prior treatment regimens.
-
How Does COVID-19 Affect People with Cancer? NCCAPS Will Help Find Out
NCI has launched the COVID-19 in Cancer Patients Study (NCCAPS), which will help answer questions about COVID-19’s impact on cancer patients. The study is now open to adults and will later be expanded to include children.
-
Updated Nutrition Facts Label Reflects Science on Diet and Health, including Cancer
On January 1, 2020, the Food and Drug Administration (FDA) began requiring food manufacturers to display updated nutrition facts labels on their product packaging. Experts from FDA and NCI discuss the update and the research that underpins the changes.