Raising the R01 Payline and Funding Other NCI Priorities
, by NCI Acting Director Dr. Douglas R. Lowy and NCI Associate Director for Finance and Legislation Patrick McGarey
NCI Acting Director Dr. Lowy and NCI Associate Director for Finance and Legislation Patrick McGarey explain why raising the R01 payline to the 15th percentile by fiscal year 2025 and supporting more R01 grant awards is a top priority for NCI. They also discuss why allocating funds for this priority must be balanced with maintaining other critical NCI programs extramural scientists rely on to support their research.
Each September, we witness a turn of the seasons as summer ends and fall begins. For NCI, September also marks the end of one fiscal year (FY) and a new opportunity to fund promising cancer research as another FY begins.
When NCI charts its financial plan each year, our foremost priority is funding the most compelling cancer research opportunities that reflect the full breadth of science: prevention and screening, diagnosis and treatment, quality of life and survivorship, and the basic biology that serves as a foundation for all these areas of cancer science. And as we develop our plan for a new FY, one of the first questions we ask is, how can our funding support more new R01 grant awards?
Research project grants, commonly known as R01 grants to academic researchers, have a long and prominent history at NCI. They are highly competitive and recognized as premier awards by the science community. In recent years, about two-thirds of funding dedicated to competing grants paid for R01 awards.
Year-by-year, some of the most significant discoveries in cancer science emerge from research by investigators who receive R01 awards. For example, during a 10-year period beginning in 2009, the Nobel Committee awarded five prizes in Physiology or Medicine to scientists who received NCI R01 grants. These results highlight why funding R01 grants is a priority for NCI.
Paylines and NCI’s 15-by-25 goal
Each FY, NCI establishes paylines for competing grant applications, setting different paylines for different grant mechanisms (R01s, R21s, etc.). When applying a payline to the applications NCI receives, NCI awards funding to nearly all grant applications with peer review scores better than or equal to the payline NCI establishes.
NCI’s ability to fund grants based on a payline is primarily influenced by two factors: our available budget and the volume of grant applications we receive. In FY 2019, the R01 payline was at the 8th percentile, a disappointingly low number caused by the unprecedented growth in the volume of NCI grant applications received in the years leading up to FY 2019.
In the NCI Annual Plan and Budget Proposal for FY 2022, NCI announced a goal of raising the R01 payline to the 15th percentile by FY 2025. This is a priority we fully embrace, and we are pleased with the progress NCI has made toward our goal. The NCI R01 payline for FY 2022, which ends on September 30, is the 11th percentile, an increase of 37% from FY 2019.
Achieving the 15-by-25 goal would allow NCI to substantially increase the number of meritorious research applications we fund—research that will fuel broad, sustained progress that supports the needs of people with cancer and those at risk of cancer. We will gain a deeper understanding of cancer biology in ways that lead to new strategies to prevent, screen for, diagnose, and treat cancer, in all its forms.
For FY 2023, our priority is to further raise the R01 payline, issue more R01 awards, and make progress on our path to the 15-by-25 goal. However, NCI often does not receive an annual appropriation before the new FY begins. When this occurs, as we explained in a previous Bottom Line Blog post, NCI begins the FY with a temporary, interim budget.
One consequence of an interim budget is that NCI cannot set paylines for the full year when the new FY begins. Like the budget we receive, the initial paylines we set are only interim in nature. Soon after the FY begins on October 1, NCI plans to announce the FY 2023 interim paylines. Eventually, we will have the opportunity to update paylines when we receive our final appropriation.
Maintaining the components of a robust National Cancer Program
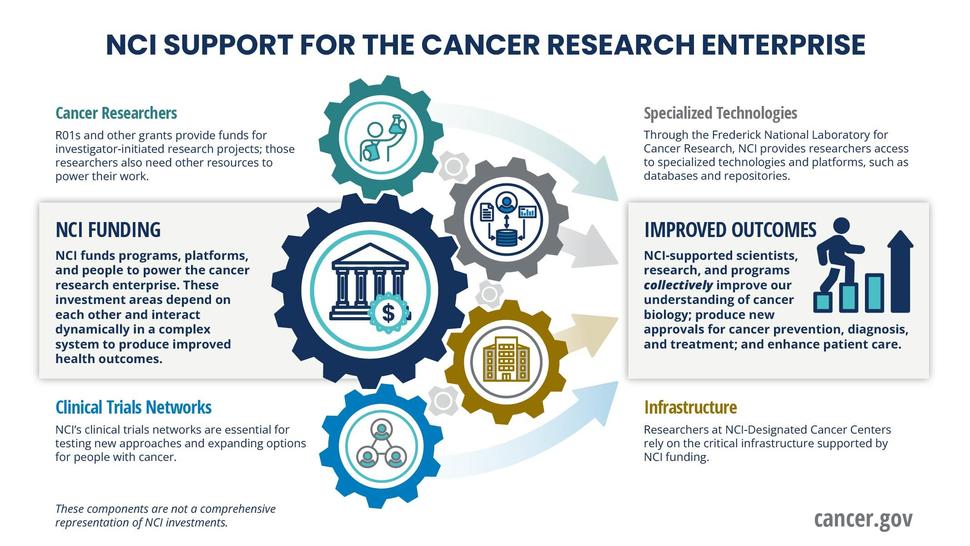
Given NCI’s priority to improve the payline for R01s, we are sometimes asked why we fund other components of the National Cancer Program, when the funding for those programs could be used to issue more R01 awards.
The brief answer is that the many components of the National Cancer Program are interwoven and highly interdependent, and reducing one component will adversely affect scientific progress elsewhere, as the following graphic illustrates. Let us offer examples of what we mean.
NCI-Designated Cancer Centers
The NCI-Designated Cancer Centers Program is the backbone of the nation’s cancer research effort. Hundreds of thousands of patients receive care or are enrolled in clinical trials at these centers each year. NCI funded the first cancer center more than 50 years ago.
NCI-Designated Cancer Centers are a dynamic engine of discovery and the nation’s single most important source of new insights into the causes of cancer and strategies to prevent, diagnose, and treat cancer. Research proposals from cancer center investigators account for about three-quarters of the investigator-initiated grants NCI issues. NCI issues more than 6,000 grant awards per year, and many of our grantees rely on the comprehensive infrastructure that cancer centers provide to support their research, which is why NCI continues to commit funding to this program.
NCI clinical trials networks
Each year, more than 75,000 new patients enroll in NCI-sponsored or supported clinical trials. These include treatment and observational trials. Half of these patients enroll in trials supported by the National Clinical Trials Network (NCTN) and the NCI Community Oncology Research Program (NCORP). Through the NCTN and NCORP networks, patients participate in studies to test new ways to screen, prevent, diagnose, and treat cancer.
These trials yield important results. Foremost among them is evidence to demonstrate the safety and effectiveness of new oncology products—evidence that leads to new US Food and Drug Administration–approved drugs, vaccines, and devices that allow patients with cancer and those at risk of cancer to lead longer, healthier lives.
Developing new treatments and improving cancer care is a core element of the NCI mission, which is why NCI gives priority to funding these networks. NCTN and NCORP provide the vital infrastructure that NCI grantees rely on to conduct clinical trials and advance their research.
Frederick National Laboratory for Cancer Research
The Frederick National Laboratory for Cancer Research (FNLCR) is a national asset and a unique resource that has been serving the cancer research community since 1971. Through FNLCR, NCI offers an array of resources to our grantees that many academic institutions could not offer on their own. Here are some examples:
The NCI Experimental Therapeutics Program (NExT) works with grantees and other extramural scientists to shorten the timeline for new drug development and speed breakthroughs of new cancer therapies. NExT focuses primarily on therapies that have the potential to meet a critical need but are unlikely to initially attract private sector investment because they are too high risk. Working with experts in fields such as structural biology and cancer cell biology, NExT advances promising therapies through the early stages of research and into clinical trials. Through the NExT program, NCI is speeding the development of therapeutics to treat ovarian cancer, mesothelioma, and childhood brain tumors.
The FNLCR Biopharmaceutical Development Program (BDP) produces novel antibodies, proteins, and other biological products to support early-stage development of promising cancer treatments. For example, to support clinical trials of a monoclonal antibody that showed promise as a treatment for neuroblastoma tumors in children, BDP manufactured the antibody that allowed the clinical trial to proceed. The monoclonal antibody, dinutuximab (Unituxin), is now the standard of care for children with certain types of neuroblastoma.
The BDP recently added the capacity to produce CAR T cells for clinical trials. For this type of immunotherapy, a cancer patient’s T cells (a type of immune system cell) are modified in the BDP laboratory so they will attack cancer cells. The product that results from BDP manufacturing becomes a therapeutic, which is then reinfused into the patient to treat their cancer. This new BDP capability will allow scientists to continue to develop and improve immunotherapy treatments for cancer patients.
The FNLCR Nanotechnology Characterization Laboratory (NCL) serves as a national resource to advance nanoscale particles into clinical application. The laboratory assists academic researchers who are studying and characterizing nanotechnology particles being developed for cancer vaccines, other cancer therapeutics, and diagnostics. NCL has evaluated more than 1,000 nanoparticle formulations for NCI grantees and extramural scientists, many of which proceeded to clinical testing.
The bottom line: Raising the R01 payline to the 15th percentile by FY 2025 and supporting more R01 grant awards is a top priority for NCI. However, allocating funds for this compelling priority must be balanced with maintaining other NCI programs extramural scientists rely on to support their research.