Developing a Low-Cost Portable Technology to Treat Cervical Precancer
, by Partha Basu, M.D., F.R.C.O.G., Ph.D., International Agency for Research on Cancer
Where authors are identified as personnel of the International Agency for Research on Cancer/ World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policies, or views of the International Agency for Research on Cancer/ World Health Organization.
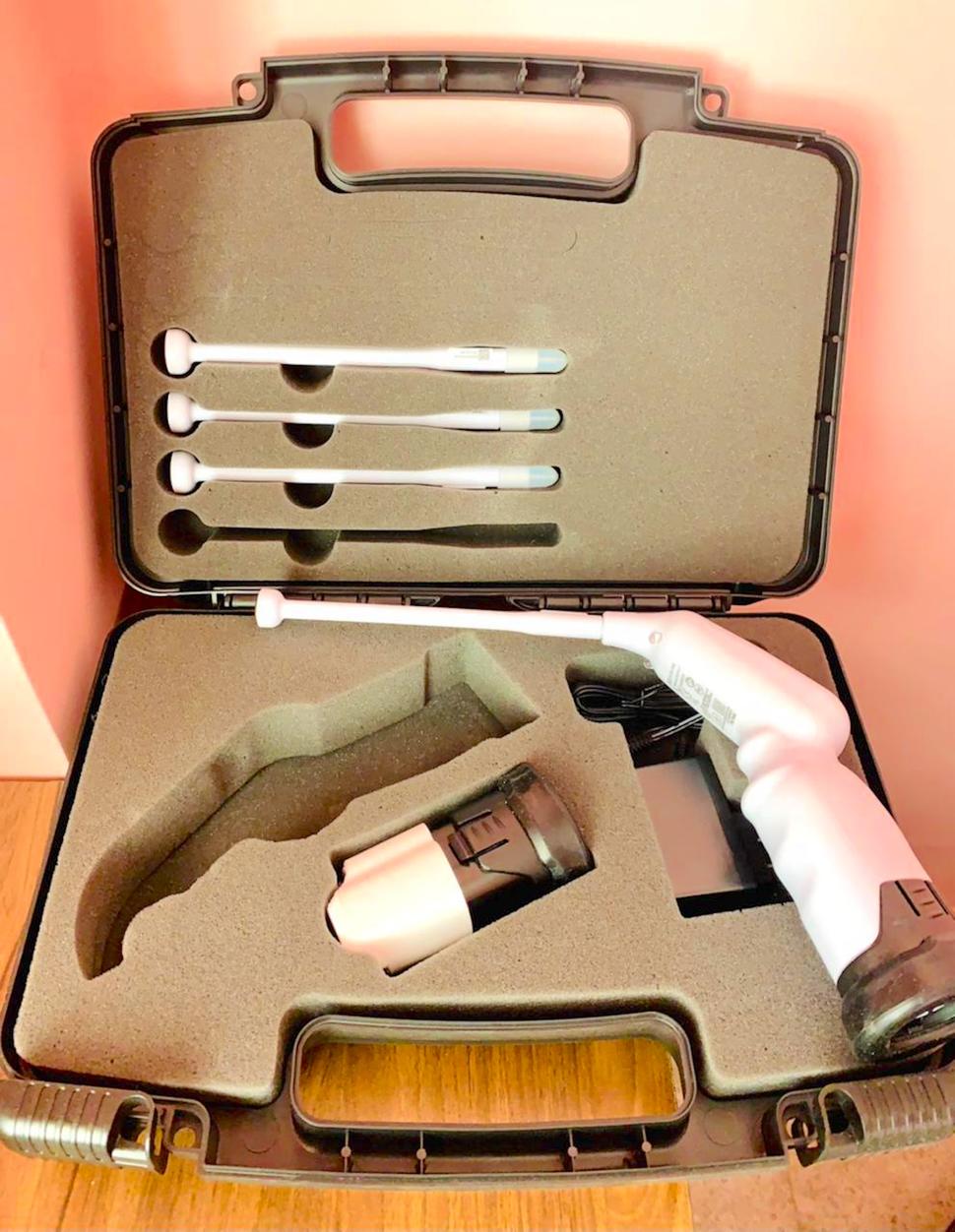
Despite being a preventable disease, cervical cancer kills more than 300,000 women every year (1). Sub-Saharan Africa and other low-resource regions around the globe, face the highest cervical cancer burden. As such, our research team at the University Teaching Hospital (UTH) in Zambia and the International Agency for Research on Cancer (IARC), collaborated to develop and test a much-needed affordable technology, the TC Thermocoagulator, a portable, battery-powered thermal ablation system, to treat cervical precancers through a National Cancer Institute (NCI) funded project. Our new technology will be highly suitable for these countries due to its portability, low cost, and ease of use by frontline health workers operating at the primary healthcare level. UTH principal investigator, Professor Groesbeck Parham, believes, "The new technology will certainly accelerate elimination of cervical cancer as a public health problem globally.”
Regular screening of apparently healthy women may detect cervical cancer at a precancerous stage. If precancers are treated appropriately, progression to invasive cancer and its consequences can be avoided. Different precancer treatment options are available including ablating the abnormal epithelium with cold or heat injury, or electrosurgically excising the abnormal epithelium. Nearly 60% to 70% of women with cervical precancers can be treated using ablative methods while the remainder would require excisional treatment—the former being simpler, safer, and less expensive (2).
For many years, the only ablative technique available was cryotherapy—using cold injury to kill the abnormal epithelial cells. The WHO strongly recommended the use of cryotherapy to treat cervical precancers in 2013, especially in limited resourced settings. The limitations of this particular technology became apparent as countries attempted to bring it to scale. Cryotherapy requires refrigerant gas (nitrous oxide or carbon dioxide) that is stored in large metal tanks. In many countries the refrigerant gas is either not available on a regular basis or is not of appropriate quality. Transporting heavy tanks to primary care facilities and storing them is challenging. Additionally, impurities in the gas may block its flow and interrupt treatment. The expense of procuring and supplying refrigerant gas further increases the overall cost of treatment. As countries with limited resources were unable to scale the treatment of cervical precancers using cryotherapy, creating an affordable, feasible, and effective technology was paramount.
Our team, comprised of researchers from IARC and their counterparts at UTH in Lusaka, Zambia—Prof. Groesbeck Parham, Prof. Mulindi Mwanahamuntu, Dr. Samson Chisele, Dr. Leeya Pinder—and Dr. Dean Wallace and his team of engineers at LIGER INC., USA, developed a lightweight, menu-driven, and low-cost thermal ablator. This reusable probe, when heated to 100 degrees centigrade and applied to the cervical epithelium can ablate the abnormal cells. Keeping in mind the requirement of resource limited settings we incorporated a rechargeable battery. Once the battery is fully charged the device can treat at least 20 women. Our device has several advantages over cryotherapy. Recurrent expenditure is minimum as no refrigerant gas, or any other consumables are required. Treatment time is significantly lower—30 to 120 seconds for thermal ablation compared to 11 minutes for cryotherapy. In fact, the machine has a built-in timer to keep track of the duration of treatment. Our probe is also designed to prevent accidental heat injury to the vagina. Despite these advantages, the safety and efficacy of the new device had to be established before the same could be recommended for large scale use. That was the specific objective of the large field trial that our team of colleagues set up in Zambia. Nested into an ongoing, government-operated, nurse-led cervical cancer prevention service platform at the primary healthcare level.
We compared safety and efficacy of the new device to that of cryotherapy in a randomized controlled trial (RCT) conducted in multiple primary health clinics in Lusaka (3). In the same study thermal ablation was compared also to large loop excision of the transformation zone (LLETZ), the most common excisional method used to treat cervical precancers. Nurses screened women with visual inspection with acetic acid (VIA) screening tests. Those with positive test results and eligible for ablative therapy were randomized in equal numbers to the three arms to be treated with either thermal ablation, cryotherapy, or LLETZ. The large study recruited a total of 3,124 women equally distributed between the three treatment arms. “I am privileged to have had the opportunity to contribute towards this research that will play a crucial role in enhancing the delivery of cervical cancer prevention services,” said Ms. Namakau Nyambe, a social worker and project coordinator from UTH. It was exciting to see that all three techniques performed equally well to treat the patients. In our study, less women complained of significant pain during treatment with thermal ablation compared to that with cryotherapy. Among HIV negative women 86.9% were successfully treated with thermal ablation. The proportion was 83.9% and 86.5% for cryotherapy and LLETZ respectively.
The study also revealed that the treatment success rate was at least 20% lower in women living with HIV (WLHV) compared to HIV-negative women, irrespective of the treatment technique used. This finding from our study is a matter of great concern. WLHV have six times higher risk of developing cervical cancer compared to HIV negative women. Globally, 5% of all cervical cancers are detected in WLHV, which explains the high cervical cancer burden in sub-Saharan Africa. Nearly 40% of WLHV in our study had treatment failure even though all were on anti-retroviral therapy. It is imperative that global public health experts and researchers take a deeper dive into the issue of high cervical precancer treatment failure among WLHV. We have seen a positive correlation between certain patterns of vaginal microbiota and treatment failure in a small sub-study nested in the Zambian RCT, and further research in the area may reveal some promising solutions.
Namakau’s words reflect the sentiment of our entire study team very well, “Seeing the significant impact of our efforts on women's health and knowing that these improvements will continue to benefit future generations brings me immense satisfaction.”