Cancer Researchers Bring Tools, Experience to COVID-19 Studies
, by Edward Winstead
In her spare time last year, Neelam Giri, M.D., joined an effort to test fellow NIH employees for SARS-CoV-2, the coronavirus that causes COVID-19. She administered the test to many employees with symptoms of COVID-19 in their cars outside the NIH Clinical Center in all kinds of weather.
For Dr. Giri, a staff clinician in NCI’s Division of Cancer Epidemiology and Genetics (DCEG), the testing was volunteer work. And in December, she became one of the first frontline workers at the Clinical Center to receive the Moderna COVID-19 vaccine.
“I’m honored to be part of this campaign to end the pandemic,” Dr. Giri said before receiving the vaccine at an event to kick off COVID-19 vaccinations among NIH employees. Her volunteer job illustrates one of the many ways that cancer researchers have been working against COVID-19.
Since the pandemic began, cancer researchers have also been contributing their expertise and resources to scientific investigations of the coronavirus. Their findings have been broad in scope, ranging from insights into how the virus enters cells to the identification of potential therapies.
For example, cancer researchers reported recently that antibodies to SARS-CoV-2 may protect people from being reinfected by the virus. The research was part of NCI’s ongoing response to the pandemic, which also includes a study of patients with cancer and COVID-19 and studies of genomic factors that influence the severity of the disease.
As the pandemic continues, the results of these and other studies could inform the prevention and treatment of COVID-19 among individuals with and without cancer, according to cancer researchers who have investigated SARS-CoV-2.
“Many cancer researchers have been able to pivot portions of their research—either in the laboratory or in the clinic—to try to better understand COVID-19 and find ways to treat the disease,” said James Gulley, M.D., Ph.D., head of the immunotherapy section of NCI’s Center for Cancer Research (CCR).
Cancer researchers are well suited to investigate COVID-19 “because we are used to dealing with complex biological problems,” Dr. Gulley continued. And some of the tools used to study how the immune system interacts with tumors can be modified to study SARS-CoV-2, he added.
Testing Biomarkers for the Severity of COVID-19
Last spring, for example, several cancer researchers in New York City shifted their focus from studying immunotherapy—treatments that help the immune system to detect and kill cancer cells—to investigating the body’s response to the coronavirus.
Sacha Gnjatic, Ph.D., of the Icahn School of Medicine at Mount Sinai and his colleagues identified proteins called cytokines that could be indicators, or biomarkers, of the severity of COVID-19 and the response to treatment. Increased blood levels of two cytokines—IL-6 and TNF-a—were associated with poor survival and severe forms of COVID-19 in a large group of hospitalized patients.
The results suggested that these cytokines could potentially guide decisions about the type of care that people with COVID-19 should receive, Dr. Gnjatic said. “Such biomarkers could be evaluated in future clinical trials,” he added.
About 10% of the patients with COVID-19 in the study also had cancer. “We are still analyzing the data to see whether there are certain factors that make these patients more likely to develop severe COVID-19 than other patients,” said Dr. Gnjatic.
He brought to the project his experience leading an NCI-sponsored initiative to develop biomarkers that doctors could use to identify patients with cancer who are likely to respond to immunotherapy drugs.
“We are interested in the interplay between tumors and the immune system,” said Dr. Gnjatic. “When COVID-19 hit, we were primed to use our research methods to investigate the pathology of the disease.”
Starting in March, Dr. Gnjatic co-led a team of researchers at Mount Sinai Hospital that created a COVID-19 research biobank. In just two months, the biobank collected blood samples from 500 patients hospitalized with COVID-19. Since then, the biobank has added samples from nearly 300 hospitalized patients, and all of these patients have been followed over time.
“We now have at least 6 months of follow-up data,” Dr. Gnjatic said. “The biobank will allow us to analyze many more biomarkers, predict patient outcomes, assess the impact of treatment, and hopefully contribute to better clinical care of patients with COVID-19.”
Investigating COVID-19 in People with Cancer
Researchers have reported that people with cancer may be at an increased risk of developing more serious forms of COVID-19.
“The prior therapies that patients with cancer have had may make them more likely to get sick from COVID-19,” said Nirali Shah, M.D., of CCR, who co-led a clinical trial testing the drug tocilizumab (Actemra) in patients with cancer and COVID-19. Cancer and certain treatments for cancer, she noted, can weaken the immune system.
Patients with cancer also tend to be older and may have risk factors linked to aggressive forms of COVID-19, noted Ziad Bakouny, M.D., of the Dana-Farber Cancer Institute, who coauthored a recent overview of cancer and COVID-19. These risk factors include certain underlying health conditions, such as diabetes and a heart condition.
“In general, patients with cancer have more severe COVID-19 symptoms at diagnosis and, unfortunately, they also have worse outcomes than patients who don’t have cancer,” Dr. Bakouny said.
More research, he continued, is needed to understand “how the biology of cancer and COVID-19 may interact in individuals with both diseases.”
Some answers may come from the NCI COVID-19 in Cancer Patients Study (NCCAPS). In this natural history study, researchers are collecting data, blood samples, and images from people with cancer and COVID-19. Participants will provide blood samples at multiple time points over a 2-year period.
“We expect that the samples and data we are collecting will help researchers to better understand many aspects of how COVID-19 is affecting patients with cancer,” said Larissa Korde, M.D., of NCI’s Cancer Therapy Evaluation Program and a leader of the NCCAPS study.
The researchers have been enrolling children and adults at some 700 sites across the country, including sites that are part of the NCI Community Oncology Research Program (NCORP). NCORP reaches patients in underserved areas, many of which have been disproportionately affected by the pandemic.
The results will complement findings from the COVID-19 and Cancer Consortium, a research study involving 125 hospitals across the country that is collecting data about people diagnosed with COVID-19 and cancer, noted Dr. Korde.
Revealing Clues to Coronavirus Infections and Treatment Possibilities
Some cancer researchers, including DCEG's Ludmila Prokunina-Olsson, Ph.D., have focused on the underlying biology of coronavirus infections.
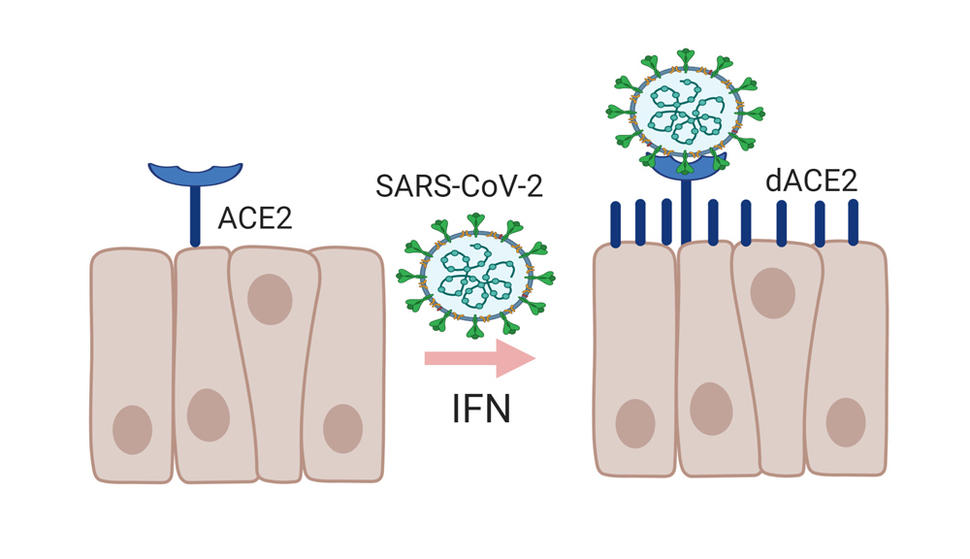
Last fall, her team described a previously unknown form of ACE2, the receptor protein used by the coronavirus to bind to and infect cells. The newly identified molecule—now called deltaACE2 (dACE2)—is shorter than the other form of ACE2 and does not appear to bind to SARS-CoV-2, which means that it is unlikely to be a gateway for viruses to enter human cells, said Dr. Prokunina-Olsson.
The researchers also found that certain cells, including some tumor cells, produce dACE2 when exposed to interferons. The body makes interferons in response to viral infections; interferons are also synthetically produced as drugs to treat cancer, infections, and other diseases. In clinical trials, researchers have been testing interferons as possible treatments for COVID-19.
In their study, Dr. Prokunina-Olsson and her colleagues found that the full-length ACE2 protein did not appear to be produced by cells in response to exposure to interferons or viruses, as some previous studies had suggested.
Taken together, the new findings suggest that exposure to viruses or interferons used for treatment may lead to the expression of dACE2 rather than the full-length form of the ACE2 receptor—and would therefore not increase the risk of cells being infected by SARS-CoV-2.
Two other groups of researchers recently confirmed the existence of dACE2 in human cells. “We are conducting additional experiments to understand why and when dACE2 is produced by normal and tumor cells—and whether differences in the expression of ACE2 and dACE2 could be important for infection,” said Dr. Prokunina-Olsson.
Profiling T-Cell Responses to the Coronavirus
Cancer researchers have also been investigating the body’s response to SARS-CoV-2, including the role that immune cells called T cells may play in fighting the infection.
“T cells can identify cells that have been infected by the virus and kill those cells,” said Dr. Gulley. “We think that studying T cells will be important for understanding the immune system’s response to SARS-CoV-2 as well as the immune response to vaccines against the virus.”
In cancer immunotherapy research, investigators routinely monitor how T cells are turned on, or activated, in response to certain proteins (antigens) on tumor cells. “We can bring this experience to the fight against COVID-19,” said Dr. Gulley.
Some of Dr. Gulley’s colleagues in CCR have done just that. A team led by Jeffrey Schlom, Ph.D., and Renee Donahue, Ph.D., has adapted tests used to profile T-cell responses to tumor antigens for studies of the coronavirus.
“As COVID-19 emerged, we modified the tests so that we could specifically measure T-cell responses against certain parts of the coronavirus, such as the spike protein on the surface of the virus and the nuclear protein,” said Dr. Donahue, of the Laboratory of Tumor Immunology and Biology.
The new technology “offers a very sophisticated way of looking at T cells and determining how active they are against certain viral proteins,” said Dr. Gulley.
The tests could be used to study COVID-19 vaccines in patients with cancer who are receiving immunotherapy, noted Dr. Donahue. “We need to learn whether COVID-19 vaccines can generate effective immune responses in patients being treated for cancer,” she added.
Understanding Inflammatory “Storms”
In some patients with severe COVID-19, the immune system mounts an overly aggressive response to the virus. When this happens, the body may produce large numbers of cytokines. By stimulating the immune system, these proteins can damage vital organs, such as the lungs and the heart, leading to death. This hyperinflammatory state is sometimes called a cytokine storm.
Uncontrolled immune responses involving cytokines can also occur in patients with cancer who receive immunotherapy drugs known as CAR T-cell therapies. In these patients, the phenomenon—called cytokine release syndrome—occurs when large amounts of cytokines are released into the blood all at once.
Such responses can be life-threatening, so patients receiving immunotherapy are routinely monitored for evidence of aggressive immune responses and treated as needed.
Although some of the same cytokines may be involved in responses to CAR T-cell therapy and to the coronavirus, the underlying biology of these responses is different, Dr. Shah explained.
“Fundamentally, what is happening with COVID-19 is that an infection leads to an inflammatory response,” said Dr. Shah.
“There may be direct or indirect injury to tissue as a result of COVID-19, and this could lead to very different immune responses,” she continued. “Also, for a host of reasons, some patients may have more of an inflammatory response than others.”
Understanding why people can have such different responses to infection with the coronavirus is the focus of ongoing investigations. For example, the NIH-led COVIDcode study is examining how genetic variants may contribute to the severity of COVID-19.
Testing Potential Treatments for COVID-19
Cancer researchers have also played a role in identifying and evaluating potential treatments for overactive immune responses associated with COVID-19. Several cancer drugs—or drugs being studied as cancer treatments—have been evaluated for this purpose.
“The results of these studies have been mixed, and more research is needed to determine which treatments may be effective,” said Dr. Bakouny, who noted that certain steroids have been shown in clinical trials to treat overactive immune responses associated with COVID-19.
One of the first cancer drugs to be evaluated for COVID-19 was acalabrutinib (Calquence). This treatment blocks the activity of a protein called Bruton’s tyrosine kinase (BTK), which plays an important role in the normal immune system.
Last March, a team led by Wyndham Wilson, M.D., and Louis Staudt, M.D., Ph.D., in CCR launched a small study to test acalabrutinib in 19 patients hospitalized with severe COVID-19.
The researchers had conducted the studies that led to acalabrutinib’s approval for certain types of lymphoma and leukemia. Some of this research had suggested that BTK inhibitors could impair the body’s immune response.
“We took the knowledge we had about the drug from our cancer studies and tried to apply that to the treatment of patients with COVID-19 who had the most dramatic immune responses,” said NCI’s Mark Roschewski, M.D., who helped conduct the study.
In the study, some of the 19 patients seemed to benefit from the drug. But in a subsequent randomized clinical trial, the drug did not improve the number of patients who were alive and free of respiratory failure, according to the maker of acalabrutinib, AstraZeneca.
Nonetheless, the research on BTK inhibitors that began during the pandemic will continue through a study called RESPOND, which is led by the National Institute of Allergy and Infectious Diseases.
“What we learn may help us understand how these inhibitors could potentially be useful for the treatment of other common inflammatory and autoimmune conditions that afflict the general population,” said the study’s lead investigator, Michail Lionakis, M.D., Sc.D., who also collaborated on the NCI-led acalabrutinib research.
An Unprecedented Pace of Scientific Discovery
Researchers have been studying coronaviruses for decades, Dr. Gulley noted, so investigators “already have a head start on identifying important questions to explore.”
He added, “The more research tools we can bring to this fight—and the more different angles we can come at this virus—the better our chances of gaining insights that will help us to more effectively treat the virus and limit its spread.”
Dr. Prokunina-Olsson said that the pace of scientific discoveries related to COVID-19 has been “unprecedented.” She undertook her study of ACE2 in response to research that had been posted online for the scientific community early in the pandemic.
The practice of sharing scientific results almost in real time fuels new studies and raises additional research questions, Dr. Prokunina-Olsson stressed.
“This process has allowed the research community to conduct follow-up studies and to refine the messages of previous publications,” she said. “What could normally take several years happened in a matter of months.”