Rucaparib Approved as Maintenance Treatment for Some Recurrent Ovarian Cancers
, by NCI Staff
The Food and Drug Administration (FDA) has expanded the approval of the targeted therapy rucaparib (Rubraca) for the treatment of women with ovarian cancer.
The approval covers the use of rucaparib as a follow-on, or maintenance, treatment for women whose ovarian cancer has returned after their initial treatment and whose tumors then shrank, at least partially, during subsequent treatment with a platinum-based chemotherapy. The approval, announced on April 6, also includes women with fallopian tube or primary peritoneal cancer.
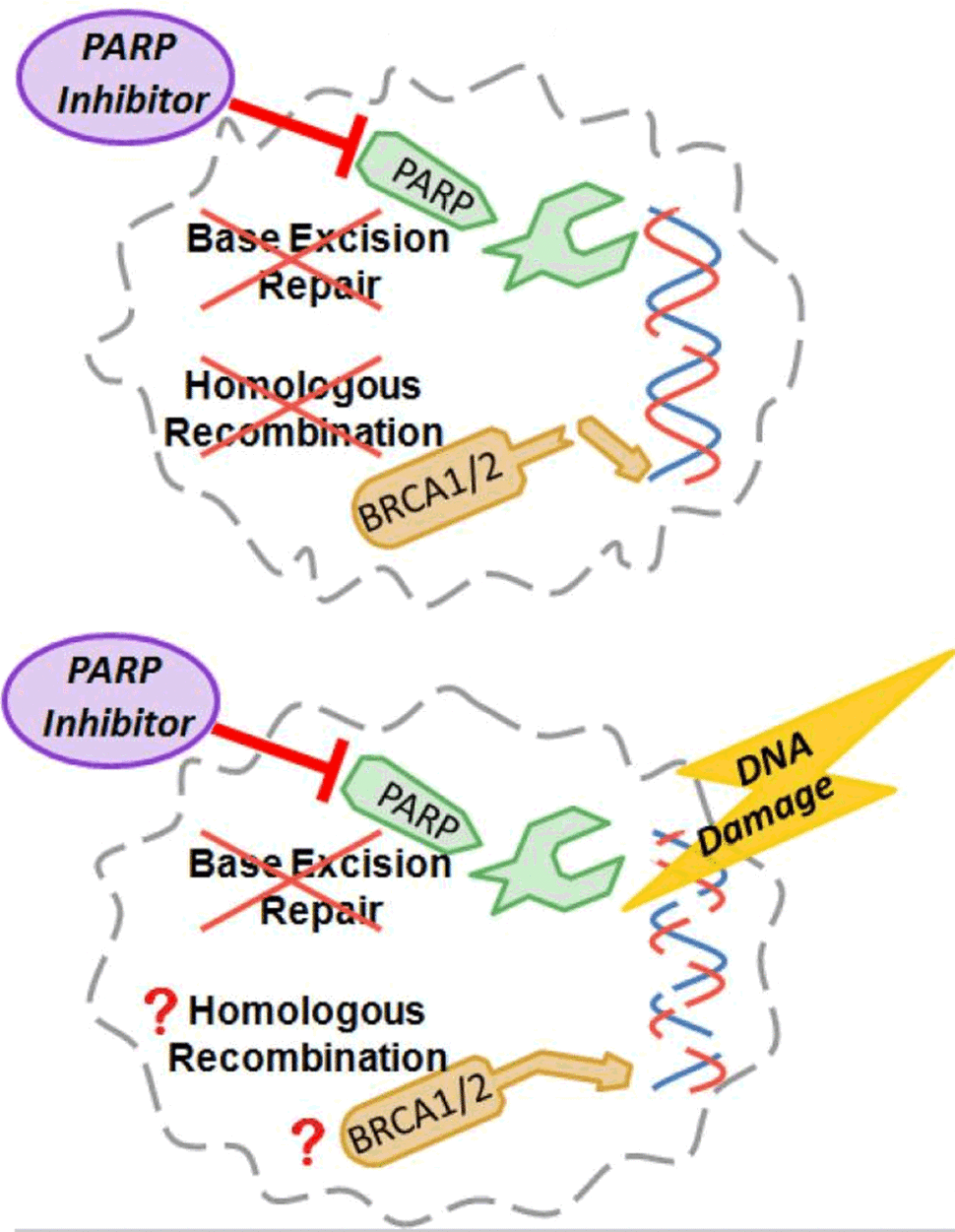
Rucaparib is a type of drug called a PARP inhibitor, which prevents cells from repairing damage to their DNA. Rucaparib joins olaparib (Lynparza) and niraparib (Zejula) as the third PARP inhibitor to be approved as a maintenance therapy for women with recurrent ovarian, fallopian tube, or primary peritoneal cancers that still respond to platinum-based chemotherapies.
What makes the rucaparib approval novel, explained Elise Kohn, M.D., head of Gynecologic Cancer Therapeutics in NCI's Division of Cancer Treatment and Diagnosis, is that it includes the approval of the first companion diagnostic test measuring possible indicators of PARP sensitivity. The test, called FoundationFocus CDxBRCA LOH, can help predict which tumors might be most likely to respond to the treatment.
Under the approval, rucaparib may be prescribed without this test. However, Dr. Kohn explained, results from it—or from other tests like it under development—may help patients and their doctors make decisions about if and what type of maintenance therapy might make the most sense, also taking into account individual health issues and treatment goals, added Dr. Kohn.
Disrupting DNA Repair in Cancer Cells
Most cells have many molecular mechanisms for repairing DNA, and PARP proteins are part of just one of these mechanisms. That means that drugs like rucaparib, which work by blocking the activity of PARP proteins, are most effective in cancer cells that already have damage to other DNA repair mechanisms, explained Robert Coleman, M.D., of the University of Texas MD Anderson Cancer Center.
Earlier clinical trials of PARP inhibitors found that they worked best in patients with mutations in the BRCA1 and BRCA2 genes, both of which play important roles in DNA repair.
However, ongoing research has suggested that PARP inhibitors may also work in tumors with other types of DNA repair defects, including a broad category of defects called homologous recombination deficiency (HRD). The companion diagnostic test approved with rucaparib tests tumors for BRCA gene mutations and for HRD.
Less Repair, More Benefit
In the phase 3 randomized trial, called ARIEL3, that led to the new approval for rucaparib, researchers led by Dr. Coleman enrolled 564 women with recurrent ovarian, fallopian tube, or primary peritoneal cancer. ARIEL 3 was funded by Clovis Oncology, Inc., the manufacturer of rucaparib.
All the women in the trial were responding, either completely or partially, to platinum-based chemotherapy. After testing participants' tumors with the companion diagnostic test, the researchers randomly assigned 375 to receive rucaparib, which is given daily as a pill, and 189 to receive a placebo. Women continued treatment until their cancer progressed, they died, or they chose to discontinue treatment because of side effects or other reasons.
Overall, the women who received rucaparib lived a longer time without their cancer progressing (progression-free survival) than women in the placebo group: 10.8 months, compared with 5.4 months.
The benefit of rucaparib was stronger in patients with DNA repair defects, as measured by the companion diagnostic test. Women with HRD tumors (defined as those who had a BRCA mutation or a mutation in a select panel of HRD-related genes) who received rucaparib lived for a median of 13.6 months without their disease progressing. Women with BRCA mutations treated with rucaparib had a median progression-free survival of 16.6 months. [Editor's note: These findings have been updated to correct errors in the original story.]
Serious side effects were more common in the women receiving rucaparib, including anemia, nausea, and headache. These side effects are common in women treated with any of the PARP inhibitors, explained Dr. Kohn. Two patients in the rucaparib group died because of secondary cancers related to treatment, compared with none in the placebo group.
The ARIEL3 researchers are continuing to follow trial participants over time to see whether treatment with rucaparib improves how long women live overall (overall survival).
Future Paths for PARP Inhibition
As clinicians become more comfortable using companion diagnostics to measure HRD and other biomarkers to gauge the potential vulnerability of a tumor to PARP inhibition, "they could end up being important decision-making tools," said Dr. Coleman.
Future trials will likely look at administering PARP maintenance therapy sooner in ovarian cancer treatment, after initial therapy (called primary maintenance), Dr. Coleman explained.
Researchers are also interested in testing PARP inhibitors as an initial, or first-line, treatment in both BRCA-positive and BRCA-negative ovarian cancers, continued Dr. Coleman.
Research in PARP inhibitors has also been expanded to include combinations with immunotherapies, such as immune checkpoint inhibitors, and with targeted therapies, like cediranib, explained Dr. Kohn. "There's a huge [effort] looking at how to leverage the activity of PARP inhibitors in combination therapies. That's the next step," she said.
Many of these trials are based on new knowledge of DNA repair mechanisms that has been gleaned from studying why some tumors are resistant to PARP inhibition, which in turn has led to the development of new drugs targeting DNA repair, she continued.
"It's a really nice circle that’s been a welcome development," concluded Dr. Kohn.