Intraocular (Uveal) Melanoma Treatment (PDQ®)–Health Professional Version
General Information About Intraocular (Uveal) Melanoma Treatment
Incidence and Mortality
Melanoma of the uveal tract (iris, ciliary body, and choroid) is rare, but it is the most common primary intraocular malignancy in adults. The mean age-adjusted incidence of uveal melanoma in the United States is approximately 4.3 new cases per million people, with no clear variation by latitude. The incidence is higher in men (4.9 cases per million) than in women (3.7 cases per million).[1] The age-adjusted incidence of this cancer has remained stable since at least the early 1970s.[1,2] U.S. incidence rates are lower than the rates of other reporting countries, which vary from about 5.3 to 10.9 cases per million. Some of the variation may be the result of differences in inclusion criteria and methods of calculation.[1]
Uveal melanoma is most often diagnosed in older individuals, with a progressively rising, age-specific incidence rate that peaks near age 70 years.[3]
Host susceptibility factors associated with the development of this cancer include:[2-4]
- White race and ethnicity.
- Light eye color.
- Fair skin.
- The ability to tan.
In view of these susceptibility factors, numerous observational studies have explored the relationship between sunlight exposure and risk of uveal melanoma. These studies have found only weak associations or yielded contradictory results.[3] Similarly, there is no consistent evidence that occupational exposure to UV light or other agents is a risk factor for uveal melanoma.[3,5]
Anatomy
Uveal melanomas can arise in the anterior (iris) or the posterior (ciliary body or choroid) uveal tract.[6] Most uveal tract melanomas originate in the choroid. The ciliary body is a less common site of origin, and the iris is the least common. The comparatively low incidence of iris melanomas has been attributed to the characteristic features of these tumors; they tend to be smaller, slower growing, and relatively dormant compared with their posterior counterparts. Iris melanomas rarely metastasize.[7] Melanomas of the posterior uveal tract generally have a more malignant histological appearance; are detected later; and metastasize more frequently than iris melanomas. The typical choroidal melanoma is a brown, elevated, dome-shaped subretinal mass. The degree of pigmentation ranges from dark brown to totally amelanotic.
Most uveal melanomas are initially completely asymptomatic. As the tumor enlarges, it may cause distortion of the pupil (iris melanoma), blurred vision (ciliary body melanoma), or markedly decreased visual acuity caused by secondary retinal detachment (choroidal melanoma). Serous detachment of the retina may occur. If extensive detachment occurs, secondary angle-closure glaucoma occasionally develops. Clinically, several lesions simulate uveal melanoma, including metastatic carcinoma, posterior scleritis, and benign tumors, such as nevi and hemangiomas.[8]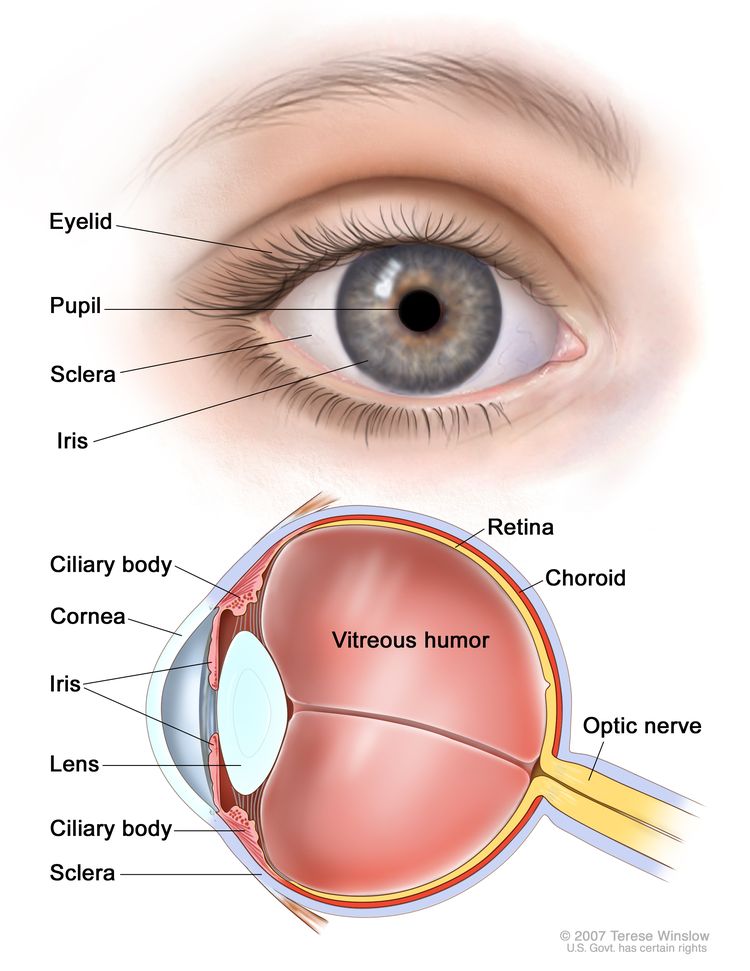
Diagnosis
Careful examination by an experienced clinician remains the most important test to diagnose intraocular melanoma. A small uveal melanoma cannot be distinguished from a nevus. Small uveal lesions are observed for growth before making a diagnosis of melanoma. Clinical findings that may help to identify melanoma include:[6]
- Orange pigment on the tumor surface.
- Subretinal fluid.
- Tumor thickness of more than 2 mm.
- Low internal reflectivity on ultrasound examination.
Ancillary diagnostic testing, including fluorescein angiography and ultrasonography, can be extremely valuable in establishing and confirming the diagnosis.[9] In a large, retrospective, single-center series of 2,514 consecutive patients with choroidal nevi, the progression rate to melanoma was 8.6% at 5 years, 12.8% at 10 years, and 17.3% at 15 years.[10]
Prognostic Factors
Several factors influence prognosis. The most important factors include:
- Cell type. For more information, see the Cellular Classification of Intraocular (Uveal) Melanoma section.
- Tumor size.
- Location of the anterior margin of the tumor.
- Degree of ciliary body involvement.
- Extraocular extension.
Several additional microscopic features can affect the prognosis of intraocular melanoma, including:
- Mitotic activity.
- Lymphocytic infiltration.
- Fibrovascular loops (possibly).
Cell type is the most commonly used predictor of outcome following enucleation. Patients with spindle-A cell melanomas have the best prognosis and patients with epithelioid cell melanomas have the least favorable prognosis.[1,4,9] Nevertheless, most tumors have an admixture of cell types, and there is no clear consensus regarding the proportion of epithelioid cells that constitutes designation of a tumor as mixed or epithelioid.[6]
Extraocular extension, recurrence, and metastasis are associated with an extremely poor prognosis, and long-term survival cannot be expected for patients with these features.[11] The 5-year mortality rate for patients with metastasis from ciliary body or choroidal melanoma is approximately 30%, compared with a rate of 2% to 3% for patients with iris melanomas.[12]
References
- Singh AD, Topham A: Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology 110 (5): 956-61, 2003. [PUBMED Abstract]
- Inskip PD, Devesa SS, Fraumeni JF: Trends in the incidence of ocular melanoma in the United States, 1974-1998. Cancer Causes Control 14 (3): 251-7, 2003. [PUBMED Abstract]
- Singh AD, Bergman L, Seregard S: Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am 18 (1): 75-84, viii, 2005. [PUBMED Abstract]
- Weis E, Shah CP, Lajous M, et al.: The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol 124 (1): 54-60, 2006. [PUBMED Abstract]
- Harris RB, Griffith K, Moon TE: Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985-1996. J Am Acad Dermatol 45 (4): 528-36, 2001. [PUBMED Abstract]
- Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 805–17.
- Yap-Veloso MI, Simmons RB, Simmons RJ: Iris melanomas: diagnosis and management. Int Ophthalmol Clin 37 (4): 87-100, 1997 Fall. [PUBMED Abstract]
- Eye and ocular adnexa. In: Rosai J: Ackerman’s Surgical Pathology. 8th ed. Mosby, 1996, pp 2449-2508.
- Albert DM, Kulkarni AD: Intraocular melanoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 2090-8.
- Shields CL, Furuta M, Berman EL, et al.: Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol 127 (8): 981-7, 2009. [PUBMED Abstract]
- Gragoudas ES, Egan KM, Seddon JM, et al.: Survival of patients with metastases from uveal melanoma. Ophthalmology 98 (3): 383-9; discussion 390, 1991. [PUBMED Abstract]
- Introduction to melanocytic tumors of the uvea. In: Shields JA, Shields CL: Intraocular Tumors: A Text and Atlas. Saunders, 1992, pp 45-59.
Cellular Classification of Intraocular (Uveal) Melanoma
Primary intraocular melanomas originate from melanocytes in the uveal tract.[1] The following four distinct cellular types are recognized in intraocular melanoma (revised Callender classification):[2]
- Spindle-A cells (spindle-shaped cells with slender nuclei and lacking visible nucleoli).
- Spindle-B cells (spindle-shaped cells with larger nuclei and distinct nucleoli).
- Epithelioid cells (larger polygonal cells with one or more prominent nucleoli).
- Intermediate cells (similar to but smaller than epithelioid cells).
Most primary intraocular melanomas contain variable proportions of epithelioid, spindle-A, and spindle-B cells (mixed-cell melanomas). Pure epithelioid-cell primary melanomas are infrequent (approximately 3% of cases).[1] In the Collaborative Ocular Melanoma Study, mixed-cell melanomas predominated (86% of cases).[3]
References
- Klintworth GK, Scroggs MW: The eye and ocular adnexa. In: Sternberg SS, ed.: Diagnostic Surgical Pathology. Lippincott Williams & Wilkins, 1999, pp 994-6.
- Grossniklaus HE, Green WR: Uveal tumors. In: Garner A, Klintworth GK, eds.: Pathobiology of Ocular Disease: A Dynamic Approach. 2nd ed. M. Dekker, 1994, pp 1423-77.
- Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol 125 (6): 745-66, 1998. [PUBMED Abstract]
Classification and Stage Information for Intraocular (Uveal) Melanoma
Tumor Size
Uveal melanoma most often assumes a nodular or dome-shaped configuration. Occasionally, tumors are flat or diffuse and involve extensive areas of the uvea with little elevation.
Tumor size classifications according to boundary lines used in a Collaborative Ocular Melanoma Study (COMS) are as follows:[1]
- Small: Range from 1.0 to 3.0 mm in apical height and largest basal diameter of 5.0 to 16.0 mm.[1]
- Medium: Range from 3.1 to 8.0 mm in apical height and a basal diameter of not more than 16.0 mm.[2]
- Large: Greater than 8.0 mm in apical height or a basal diameter more than 16.0 mm, when the apical height is at least 2.0 mm.
Although most ocular melanomas have a raised configuration, about 5% grow in a diffuse pattern that also may have prognostic significance. The tumors have a horizontal, flat-growth pattern, with the thickness measuring approximately 20% or less than the greatest basal dimension. This uncommon variant of uveal melanoma seems to be associated with a poorer prognosis, particularly when the diameter is large and the margins are poorly defined.[3]
In clinical practice, the tumor base may be estimated in average optic disc diameters (1 dd = 1.5 mm). The average elevation may be estimated in diopters (3 diopters = 1 mm). Other techniques, such as ultrasonography, are used to provide more accurate measurements.
An important function of ophthalmic ultrasonography is the detection of extrascleral extension.[4,5] Extrascleral extension measuring 2 mm or more in thickness can be demonstrated, provided it is located behind the equator where the intraocular tumor, sclera, and adjacent orbital fat are readily imaged.[6] Orbital extraocular extension of choroidal melanoma may be found in eyes with medium and large tumors, but it is very rare in eyes with small melanomas.
Metastatic Disease
Systemic metastases are evident in only 2% to 3% of patients at the time of diagnosis of the primary ocular melanoma.[7] Because the uveal tract is a vascular structure without lymphatic channels, tumor spread occurs principally by local extension and by dissemination through the bloodstream.[7] Lymphatic spread is rare but may occur after local extension into the conjunctiva and its lymphatics.[8] Given the rarity of nodal metastases, sentinel node biopsies of nonclinically involved nodes are not done as part of the staging procedure.[7]
Systemic metastases are generally hematogenous in origin, and the first site identified is usually the liver.[9] Lung, bone, and subcutaneous sites are also common.[9] In the COMS trials, the liver was the only site of detectable metastasis in 46% of patients with metastases reported during follow-up or at the time of death; 43% had metastases diagnosed in the liver and other sites.[9] In patients with a history of ocular melanoma who present with hepatic metastases of unknown origin, metastatic melanoma is considered in the differential diagnosis.
It is particularly unusual for choroidal melanomas of any size to invade the optic nerve or its meninges.[10] Metastasis of choroidal melanoma to the contralateral choroid is also rare.[9,11]
Staging
American Joint Committee on Cancer (AJCC) stage groupings and definitions of TNM
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define melanoma of the uveal tract.[7]
As in the seventh edition of the AJCC Cancer Staging Manual, there is no staging system for iris melanomas in the eighth edition. However, TNM should still be recorded for this site and histology combination.
| T Category | T Criteria |
|---|---|
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | |
| bIris melanomas originate from, and are predominantly located in, this region of the uvea. If less than half the tumor volume is located within the iris, the tumor may have originated in the ciliary body, and consideration should be given to classifying it accordingly. | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| T1 | Tumor limited to the iris. |
| –T1a | Tumor limited to the iris, not more than 3 clock hours in size. |
| –T1b | Tumor limited to the iris, more than 3 clock hours in size. |
| –T1c | Tumor limited to the iris with secondary glaucoma. |
| T2 | Tumor confluent with or extending into the ciliary body, choroid, or both. |
| –T2a | Tumor confluent with or extending into the ciliary body, without secondary glaucoma. |
| –T2b | Tumor confluent with or extending into the ciliary body and choroid, without secondary glaucoma. |
| –T2c | Tumor confluent with or extending into the ciliary body, choroid, or both, with secondary glaucoma. |
| T3 | Tumor confluent with or extending into the ciliary body, choroid, or both, with scleral extension. |
| T4 | Tumor with extrascleral extension. |
| –T4a | Tumor with extrascleral extension ≤5 mm in largest diameter. |
| –T4b | Tumor with extrascleral extension >5 mm in largest diameter. |
| N Category | N Criteria |
|---|---|
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node involvement. |
| N1 | Regional lymph node metastases or discrete tumor deposits in the orbit. |
| –N1a | Metastasis in one or more regional lymph node(s). |
| –N1b | No regional lymph nodes are positive, but there are discrete tumor deposits in the orbit that are not contiguous to the eye (choroidal and ciliary body). |
| M Category | M Criteria |
|---|---|
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | |
| M0 | No distant metastasis by clinical classification. |
| M1 | Distant metastasis. |
| –M1a | Largest diameter of the largest metastasis ≤3.0 cm. |
| –M1b | Largest diameter of the largest metastasis 3.1–8.0 cm. |
| –M1c | Largest diameter of the largest metastasis ≥8.1 cm. |
| Category | Tumor Size |
|---|---|
| aAdapted from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | |
| 1 | Tumor is ≤12 mm in diameter and ≤3 mm in thickness; or |
| Tumor is ≤9 mm in diameter and 3.1–6 mm in thickness. | |
| 2 | Tumor is 12.1–18 mm in diameter and ≤3 mm in thickness; or |
| Tumor is 9.1–15 mm in diameter and 3.1– 6 mm in thickness; or | |
| Tumor is ≤12 mm in diameter and 6.1–9 mm in thickness. | |
| 3 | Tumor is 15.1–18 mm in diameter and 3.1–6 mm in thickness; or |
| Tumor is 12.1–18 mm in diameter and 6.1–9 mm in thickness; or | |
| Tumor is ≤18 mm in diameter and 9.1–12 mm in thickness; or | |
| Tumor is ≤15 mm in diameter and 12.1–15 mm in thickness. | |
| 4 | Tumor is >18 mm in diameter and may be any thickness; or |
| Tumor is 15.1–18 mm in diameter and >12 mm in thickness; or | |
| Tumor is ≤15 mm in diameter and >15 mm in thickness. | |
| Stage | TNM | Description |
|---|---|---|
| M = distant metastasis; N = regional lymph node; T = primary tumor. | ||
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | ||
| b1) Primary ciliary body and choroidal melanomas are classified according to four tumor-size categories based on thickness and diameter. See Table 4. 2) In clinical practice, the largest tumor basal diameter may be estimated in optic disc diameters (DD) (average: 1 DD = 1.5 mm), and tumor thickness may be estimated in diopters (average: 2.5 diopters = 1 mm). Ultrasonography and fundus photography are used to provide more accurate measurements. 3) When histopathological measurements are recorded after fixation, tumor diameter and thickness may be underestimated because of tissue shrinkage. | ||
| I | T1a, N0, M0 | –T1a = Tumor size category 1 without ciliary body involvement and extraocular extension. |
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | ||
| b1) Primary ciliary body and choroidal melanomas are classified according to four tumor-size categories based on thickness and diameter. See Table 4. 2) In clinical practice, the largest tumor basal diameter may be estimated in optic disc diameters (DD) (average: 1 DD = 1.5 mm), and tumor thickness may be estimated in diopters (average: 2.5 diopters = 1 mm). Ultrasonography and fundus photography are used to provide more accurate measurements. 3) When histopathological measurements are recorded after fixation, tumor diameter and thickness may be underestimated because of tissue shrinkage. | ||
| IIA | T1b–d, N0, M0 | –T1b = Tumor size category 1 with ciliary body involvement. |
| –T1c = Tumor size category 1 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| –T1d = Tumor size category 1 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. | ||
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| T2a, N0, M0 | –T2a = Tumor size category 2 without ciliary body involvement and extraocular extension. | |
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| IIB | T2b, N0, M0 | –T2b = Tumor size category 2 with ciliary body involvement. |
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| T3a, N0, M0 | –T3a = Tumor size category 3 without ciliary body involvement and extraocular extension. | |
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | ||
| b1) Primary ciliary body and choroidal melanomas are classified according to four tumor-size categories based on thickness and diameter. See Table 4. 2) In clinical practice, the largest tumor basal diameter may be estimated in optic disc diameters (DD) (average: 1 DD = 1.5 mm), and tumor thickness may be estimated in diopters (average: 2.5 diopters = 1 mm). Ultrasonography and fundus photography are used to provide more accurate measurements. 3) When histopathological measurements are recorded after fixation, tumor diameter and thickness may be underestimated because of tissue shrinkage. | ||
| IIIA | T2c–d, N0, M0 | –T2c = Tumor size category 2 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. |
| –T2d = Tumor size category 2 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. | ||
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| T3b–c, N0, M0 | –T3b = Tumor size category 3 with ciliary body involvement. | |
| –T3c = Tumor size category 3 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| T4a, N0, M0 | –T4a = Tumor size category 4 without ciliary body involvement and extraocular extension. | |
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| IIIB | T3d, N0, M0 | –T3d = Tumor size category 3 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. |
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| T4b–c, N0, M0 | –T4b = Tumor size category 4 with ciliary body involvement. | |
| –T4c = Tumor size category 4 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| IIIC | T4d–e, N0, M0 | –T4d = Tumor size category 4 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. |
| –T4e = Any tumor size category with extraocular extension >5 mm in largest diameter. | ||
| N0 = No regional lymph node involvement. | ||
| M0 = No distant metastasis by clinical classification. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 805–17. | ||
| b1) Primary ciliary body and choroidal melanomas are classified according to four tumor-size categories based on thickness and diameter. See Table 4. 2) In clinical practice, the largest tumor basal diameter may be estimated in optic disc diameters (DD) (average: 1 DD = 1.5 mm), and tumor thickness may be estimated in diopters (average: 2.5 diopters = 1 mm). Ultrasonography and fundus photography are used to provide more accurate measurements. 3) When histopathological measurements are recorded after fixation, tumor diameter and thickness may be underestimated because of tissue shrinkage. | ||
| IV | Any T, N1, M0 | TX = Primary tumor cannot be assessed. |
| T0 = No evidence of primary tumor. | ||
| T1 = Tumor size category 1. | ||
| –T1a = Tumor size category 1 without ciliary body involvement and extraocular extension. | ||
| –T1b = Tumor size category 1 with ciliary body involvement. | ||
| –T1c = Tumor size category 1 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| –T1d = Tumor size category 1 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. | ||
| T2 = Tumor size category 2. | ||
| –T2a = Tumor size category 2 without ciliary body involvement and extraocular extension. | ||
| –T2b = Tumor size category 2 with ciliary body involvement. | ||
| –T2c = Tumor size category 2 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| –T2d = Tumor size category 2 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. | ||
| T3 = Tumor size category 3. | ||
| –T3a = Tumor size category 3 without ciliary body involvement and extraocular extension. | ||
| –T3b = Tumor size category 3 with ciliary body involvement. | ||
| –T3c = Tumor size category 3 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| –T3d = Tumor size category 3 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. | ||
| T4 = Tumor size category 4. | ||
| –T4a = Tumor size category 4 without ciliary body involvement and extraocular extension. | ||
| –T4b = Tumor size category 4 with ciliary body involvement. | ||
| –T4c = Tumor size category 4 without ciliary body involvement but with extraocular extension ≤5 mm in largest diameter. | ||
| –T4d = Tumor size category 4 with ciliary body involvement and extraocular extension ≤5 mm in largest diameter. | ||
| –T4e = Any tumor size category with extraocular extension >5 mm in largest diameter. | ||
| N1 = Regional lymph node metastases or discrete tumor deposits in the orbit. | ||
| –N1a = Metastasis in one or more regional lymph nodes(s). | ||
| –N1b = No regional lymph nodes are positive, but there are discrete tumor deposits in the orbit that are not contiguous to the eye. | ||
| M0 = No distant metastasis by clinical classification. | ||
| Any T, Any N, M1a–c | Any T = See descriptions above in this table, stage IV, Any T, N1, M0. | |
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node involvement. | ||
| N1 = Regional lymph node metastases or discrete tumor deposits in the orbit. | ||
| –N1a = Metastasis in one or more regional lymph node(s). | ||
| –N1b = No regional lymph nodes are positive, but there are discrete tumor deposits in the orbit that are not contiguous to the eye (choroidal and ciliary body). | ||
| M1 = Distant metastasis. | ||
| –M1a = Largest diameter of the largest metastasis ≤3.0 cm. | ||
| –M1b = Largest diameter of the largest metastasis 3.1–8.0 cm. | ||
| –M1c = Largest diameter of the largest metastasis ≥8.1 cm. | ||
Prognostic features
There are several key prognostic features that are important to collect in malignant melanoma of the uvea, even though they are not included in staging algorithms. These include:[7]
Molecular features
- Chromosomal alterations.
- Chromosome 3 status (loss or no loss, complete or partial).
- Chromosome 6p status (gain or no gain).
- Chromosome 8q status (gain or no gain).
Indicate:
- Technique used for assessing chromosome status may include:
- Karyotyping.
- Fluorescence in situ hybridization.
- Comparative genomic hybridization.
- Loss of heterozygosity using DNA polymorphism analysis (e.g., single nucleotide polymorphism, microsatellite).
- Other.
- How specimen was obtained may include:
- Enucleation.
- Local resection.
- Biopsy.
- Fine-needle aspiration biopsy.
- For needle biopsies, whether cytopathological evaluation was performed to confirm the presence of tumor cells.
- Technique used for assessing chromosome status may include:
- Gene-expression profile: class 1 or class 2.
Indicate:
- Technique used for gene-expression profiling may include:
- Microarray.
- Pathological complete response.
- How specimen was obtained may include:
- Enucleation.
- Local resection.
- Biopsy.
- Fine-needle aspiration biopsy.
- For needle biopsies, whether cytopathological evaluation was performed to confirm the presence of tumor cells.
- Technique used for gene-expression profiling may include:
Clinical and histopathological features
- Clinical.
- Positron emission tomography/computed tomography.
- Fluorine F 18-fludeoxyglucose standardized uptake values (higher values in primary tumor may be associated with shorter survival).
- Confocal indocyanine green angiography.
- Identification of complex monocirculatory patterns (i.e., loops, networks, arcs with branching, parallel with cross-linking or a combination of these patterns may be associated with shorter survival).
- Positron emission tomography/computed tomography.
- Histopathological.
- Mitotic count.
- Number of mitotic figures per 40 high-power fields (typical field area 0.15–0.19 mm2, higher counts are associated with shorter survival).
- Mean diameter of the ten largest nucleoli.
- Mean of the longest nucleoli (MLN) is measured along a central 5-mm long strip, e.g., after silver staining (larger values are associated with shorter survival).
- Presence of extravascular matrix patterns.
- Loops.
- Absent.
- Present (shorter survival).
- Loops forming networks.
- Absent.
- Present (shorter survival).
- Other complex patterns (arcs with branching, parallel with cross-linking; absent or present).
The patterns are assessed with light microscopy under a dark green filter after staining with periodic-acid Schiff without counterstain.
- Loops.
- Microvascular density.
- Number of immunopositive elements labeled with markers for vascular endothelial cells (e.g., CD34 epitope, factor VIII-related antigen) in areas of densest vascularization (typical field area 0.31 mm2, higher counts are associated with shorter survival).
- Insulin-like growth factor 1 receptor (IGF1-R).
- Percentage of immunopositive tumor cells (high expression is associated with shorter survival).
- Tumor-infiltrating lymphocytes.
- Few (longest survival).
- Moderate numbers.
- Many (shortest survival).
- Tumor-infiltrating macrophages.
- Few (longest survival).
- Moderate numbers.
- Many (shortest survival).
The number can be compared with standard photographs.[12]
- HLA class I expression.
- Percentage of immunopositive tumor cells (low expression is associated with longer survival).
- Mitotic count.
References
- Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 115 (12): 1537-44, 1997. [PUBMED Abstract]
- Diener-West M, Earle JD, Fine SL, et al.: The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, II: characteristics of patients enrolled and not enrolled. COMS Report No. 17. Arch Ophthalmol 119 (7): 951-65, 2001. [PUBMED Abstract]
- Shields CL, Shields JA, De Potter P, et al.: Diffuse choroidal melanoma. Clinical features predictive of metastasis. Arch Ophthalmol 114 (8): 956-63, 1996. [PUBMED Abstract]
- Scott IU, Murray TG, Hughes JR: Evaluation of imaging techniques for detection of extraocular extension of choroidal melanoma. Arch Ophthalmol 116 (7): 897-9, 1998. [PUBMED Abstract]
- Romero JM, Finger PT, Iezzi R, et al.: Three-dimensional ultrasonography of choroidal melanoma: extrascleral extension. Am J Ophthalmol 126 (6): 842-4, 1998. [PUBMED Abstract]
- Echography (ultrasound) procedures for the Collaborative Ocular Melanoma Study (COMS), Report no. 12, Part I. J Ophthalmic Nurs Technol 18 (4): 143-9, 1999 Jul-Aug. [PUBMED Abstract]
- Uveal melanoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 805–17.
- Dithmar S, Diaz CE, Grossniklaus HE: Intraocular melanoma spread to regional lymph nodes: report of two cases. Retina 20 (1): 76-9, 2000. [PUBMED Abstract]
- Diener-West M, Reynolds SM, Agugliaro DJ, et al.: Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol 123 (12): 1639-43, 2005. [PUBMED Abstract]
- Shields CL, Santos MC, Shields JA, et al.: Extraocular extension of unrecognized choroidal melanoma simulating a primary optic nerve tumor: report of two cases. Ophthalmology 106 (7): 1349-52, 1999. [PUBMED Abstract]
- Singh AD, Shields JA, Shields CL, et al.: Choroidal melanoma metastatic to the contralateral choroid. Am J Ophthalmol 132 (6): 941-3, 2001. [PUBMED Abstract]
- Mäkitie T, Summanen P, Tarkkanen A, et al.: Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci 42 (7): 1414-21, 2001. [PUBMED Abstract]
Treatment Option Overview for Intraocular (Uveal) Melanoma
Observation
Patients with iris melanomas have relatively good outcomes, with 5-year survival rates exceeding 95%. Iris melanomas are predominantly of the spindle-cell type and are usually smaller in size than posterior melanomas because they are detected earlier. Conservative management is generally advocated whenever possible, but surgical intervention may be justified with unequivocal tumor growth or with extensive disease at initial examination.
The management of small choroidal melanomas is controversial, and it is not clear whether treating small tumors prevents metastasis.[1] The natural history of small choroidal melanomas is poorly understood. Small, pigmented, choroidal lesions cannot always be differentiated reliably on examination. Growth is a presumed indicator of malignant potential.[2] The likelihood of progression from the time of diagnosis to the time when tumor growth warrants treatment has not been well characterized. Some ophthalmologists advocate observation. This course has been justified on several grounds, including the difficulty of establishing a correct diagnosis, the lack of any documented efficacy for globe-conserving treatments, and concerns for severe treatment-related morbidity. Others have advocated earlier therapeutic intervention.[1,3,4]
Although patients diagnosed with small choroidal tumors were not eligible for participation in the Collaborative Ocular Melanoma Study (COMS), these patients could participate in a prospective follow-up study to evaluate the natural history of small lesions. The 2-year tumor growth estimates were 21%, and the 5-year tumor growth estimates were 31%.[5] Clinical risk factors associated with tumor growth included:[3,5]
- Increased tumor thickness.
- Presence of subretinal fluid.
- Orange pigmentation.
- Absence of drusen.
- Absence of retinal pigment.
- Margin at the optic disc.
- Epithelial changes surrounding the tumor.
Surgery
The selection of treatment depends on the following:
- Site of origin (choroid, ciliary body, or iris).
- Size and location of the lesion.
- Age of the patient.
- Occurrence of extraocular invasion, recurrence, or metastasis.
Enucleation
In the past, enucleation (eye removal) was the standard treatment for primary choroidal melanoma, and it is still used when large tumors are present. However, enucleation has been largely replaced by radiation therapy (i.e., brachytherapy with radioactive plaques or external-beam, charged-particle radiation therapy) to spare the affected eye.[6,7]
Pre-enucleation external-beam radiation therapy (EBRT)
A randomized trial evaluated the role of pre-enucleation EBRT for large choroidal tumors that required enucleation. The study found that EBRT had no impact on overall survival (OS).[8,9][Level of evidence A1] A COMS trial included 1,003 patients with large choroidal melanomas (≥2 mm in height and ≥16 mm in diameter, or ≥10 mm in height irrespective of diameter, or ≥8 mm in height and border <2 mm from the optic disc) with no known metastases. Patients were randomly assigned to receive enucleation alone or enucleation after preoperative external photon-beam radiation from cobalt 60 or accelerators (20 Gy in 5 daily fractions) to the orbit and globe.[8,9] Through 10 years of follow-up, the median survival in both arms was approximately 7 years, and the 10-year all-cause mortality rate was 61% in both arms (relative risk [RR]death, 1.00; 95% confidence interval [CI], 0.85–1.18). Metastasis-free survival was also nearly identical in both arms.
Transscleral local resection
Eye-sparing transscleral local resection plays a limited role in the management of uveal melanoma. It is used in patients with large choroidal and ciliary body tumors who are not candidates for radiation therapy but are highly motivated to retain their eye.[10-12] The procedure is technically demanding and is generally performed only in centers with specialized expertise in this surgery. There is a substantial risk of retinal detachment, intraocular bleeding, and complications associated with the anesthesia-induced hypotension used to decrease the risk of bleeding. Either adjuvant brachytherapy or neoadjuvant proton-beam therapy are administered. Experience is limited to retrospective, single-center, case series.[10-12][Level of evidence C3]
Surgical resection of metastases
Surgical resection of metastases from ocular melanoma has been reported in case series of highly selected patients with occasional favorable outcomes.[13,14] However, the favorable outcomes may be the result of strong patient-selection factors, and the role of resection in this setting is unclear.[13,14][Level of evidence C3]
Radiation Therapy
Episcleral brachytherapy using plaques containing small radioactive seeds is the most common form of radiation used in the management of intraocular melanoma. Iodine I 125 (125I), cobalt Co 60 (60Co), palladium Pd 103 (103Pd), iridium Ir 192 (192Ir), and ruthenium Ru 106 (106Ru) are examples of radioactive isotopes used in the brachytherapy plaques. Isotopes with relatively low photon and electron emissions (125I, 103Pd, and 106Ru) are more easily shielded to reduce the exposure to adjacent normal tissues, and 125I is probably the most commonly used radioisotope.[15] Although plaque radiation therapy allows preservation of the eye, visual acuity is frequently lost over time.
In a case series of 1,106 patients who were treated with plaque radiation therapy for uveal melanoma and who had an initial acuity of at least 20/100, 68% developed poor acuity (i.e., 20/200 or worse) within 10 years.[16]
Factors associated with worse acuity outcomes included:[16]
- Age older than 60 years.
- Diminished baseline acuity.
- Diabetes.
- Increased tumor size and thickness.
- Location near the fovea or optic disc.
- Type of isotope therapy (106Ru, 60Co, or 192Ir vs. 125I).
125I brachytherapy yields equivalent overall and melanoma metastasis-specific survival rates to enucleation for medium-sized melanomas.[17][Level of evidence A1] The randomized COMS Medium Tumor Trial compared 125I episcleral-plaque brachytherapy (85 Gy at 0.42–1.06 Gy/hr) with enucleation in 1,317 patients with medium-sized choroidal tumors (tumor height 2.5–10.0 mm and tumor diameter ≤16.0 mm that were not contiguous with the optic disc).[17] Eighty-five percent of the patients treated with 125I brachytherapy retained their eye for 5 years or more, and 37% of them had visual acuity better than 20/200 in the irradiated eye 5 years after treatment.[17] No statistically significant differences in mortality were observed between the two study arms after 12 years of follow-up, either for death from all causes or death with histopathologically confirmed melanoma metastasis.[18] The 5- and 10-year all-cause mortality rates were 19% and 35% in both study arms. The cumulative all-cause mortality rate at 12 years was 43% in the 125I arm versus 41% in the enucleation arm (RR, 1.04; 95% CI, 0.86–1.24). The 5-year metastasis-specific mortality rates were 13% in both arms; at 10 years, the rates were 21% and 22% (RR for metastasis-specific mortality, 1.07; 95% CI, 0.81–1.41 through 12 years).
In a companion study within the COMS, 209 patients were prospectively assessed for quality of life during the first 5 years of follow-up.[19] Both study groups reported increasing difficulty with vision-oriented daily activities and ocular pain as time elapsed. Most measures of visual function were similar between the two groups. However, there were statistically significant differences favoring the brachytherapy group in comfort with driving for the first year after therapy and in reported peripheral vision for the first 2 years after therapy. These differences disappeared by year 5 of follow-up.[19][Level of evidence A3]
Charged-particle EBRT (using protons, carbon ions, or helium ions) is the other major form of radiation therapy used in the management of ocular melanomas.[20-23] This form of radiation therapy requires sophisticated equipment available only at selected centers. In addition, charged-particle EBRT involves patient cooperation during treatment (e.g., voluntarily fixating the eye on a particular point so the tumor is positioned properly in the radiation beam). A lower risk of early and late local radiation failures has been reported after charged-particle EBRT than after the use of brachytherapy, possibly resulting from differences in dose distribution in the two techniques.[20][Levels of evidence B3 and C3]
In a single-center, single-surgeon study, 184 patients with uveal melanomas smaller than 15 mm in diameter and smaller than 10 mm in thickness were randomly assigned to receive 125I brachytherapy versus helium ion radiation (to an estimated dose of 70 Gy equivalents in 5 fractions over 7 to 11 days in each arm).[24] The local tumor regrowth rate by 4 years was 13.3% in the brachytherapy arm compared with 0% in the helium ion arm (P < .001). However, the rates of metastasis, death from metastasis, and overall mortality were similar in both arms.[24][Level of evidence B3]
Because of its dose distribution, charged-particle irradiation can be better used than plaque brachytherapy to treat larger tumors and tumors closer to the fovea or optic disc. A large, single-center, single-surgeon series of 2,069 patients treated with proton-beam therapy had an actuarial local control rate of 95% (95% CI, 93%–96%) at 15 years. The cumulative rate of enucleation was 16% (95% CI, 13%–20%). Enucleations were primarily the result of neovascular glaucoma (46% of enucleations), blind uncomfortable eyes (31% of enucleations), or local recurrence (23% of enucleations). As with plaque radiation, risk factors for deterioration in visual acuity after charged-particle radiation were tumor size, location near the fovea or optic disc, baseline acuity, and underlying diabetes.[21]
Similarly, another large, single-center, single-surgeon, consecutive series of 886 patients treated with proton-beam irradiation reported a local control rate of 92.1% (95% CI, 89.8%–94.6%) and ocular conservation rate of 87.3% (95% CI, 83.9%–90.9%) at 10 years.[22][Level of evidence C3] The actuarial OS rate at 10 years was 64.1% (95% CI, 59.5%–69.0%).
In a single-center, phase I/II study of 57 evaluable patients treated with carbon ion-beam irradiation and followed for a median of 26 months, 26 patients developed neovascular glaucoma or severe eye pain from increased intraocular pressure, and 3 patients underwent enucleation. One patient had a local tumor recurrence.[23]
In an attempt to lower the complication rate and improve functional outcomes, one study compared a decreased dose of 50 cobalt Gy equivalents (CGE) with 70 CGE proton beam (each delivered in 5 fractions, usually within a 7-day period). Patients (n = 188) with tumors smaller than 15 mm in diameter and smaller than 5 mm in height, which were located near the optic disc or macula, were randomly assigned to the two doses in a double-masked study design. At 5 years, there were no statistically significant differences in local tumor control, rate of metastasis, visual acuity, or complication rates. However, the visual fields were better in the 50-CGE group.[25][Level of evidence B3]
As noted in the Surgery section, the role of pre-enucleation external photon-beam radiation therapy has been tested in a randomized trial and has shown no impact on OS for large choroidal tumors treated with enucleation.[8,9]
External-beam–photon-beam (gamma-ray) radiation therapy with gamma-knife stereotactic radiation surgery as a single-fraction [26] or fractionated stereotactic radiation [27,28] is being investigated as an alternative to brachytherapy or charged-beam radiation for posterior uveal melanomas, particularly for tumors too large or too close to the optic disc or macula to treat with brachytherapy. Because the dose rate of radiation has a slower delivery time than is the case with charged particles, specialized techniques are used to immobilize the eye [26] or to avoid delivery of the photons while the eye is moving or closed.[28] Experience is more limited with external-beam–photon therapy than for either brachytherapy or charged-particle EBRT, and there are no controlled comparisons with either of the other techniques. Early results from single-center series suggest similar levels of local tumor control and eye retention rates, but patient-selection factors may play a role.[28][Level of evidence C3]
Transpupillary Thermotherapy
Transpupillary thermotherapy (TTT) directs an infrared laser, usually at a wavelength of 810 nm, through a dilated pupil in one or more sessions to induce heat necrosis of uveal melanomas. This method carries the theoretical advantage of high-precision destruction of tumor tissue under direct visualization. However, TTT has important limitations that confine its use to specific circumstances.[1,29] The limited ability of TTT to penetrate thick tumors with sufficient energy restricts its use to small melanomas or tumors of a size that some ophthalmologists recommend for follow-up without any initial therapy. For more information, see the Observation section. When used as the primary therapy, there are relatively high rates of local recurrence and retinal vascular damage. Recurrence rates are particularly high when the tumor abuts the optic nerve and overhangs the optic disc.[1][Level of evidence C3]
In a single-center study, 95 patients with small choroidal melanomas (diameter <10 mm and thickness <3.5 mm) were randomly assigned to receive either TTT or 125I brachytherapy (100 Gy).[30] The tumor regression rate was 92% in the TTT arm and 98% in the 125I arm (P = .4). With a mean follow-up time of 56.2 months, there were four recurrences in the TTT arm and one in the 125I arm. However, the study was too small to provide clear information on efficacy differences.
TTT is also under evaluation as an adjunct to primary therapy with proton-beam radiation. In large uveal melanomas, proton-beam therapy is associated with exudative, inflammatory, and glaucomatous complications that may require enucleation. In a single-center trial, 151 patients with uveal melanomas at least 7 mm thick or at least 15 mm in diameter were randomly assigned to receive proton-beam radiation (60 CGEs over four daily fractions) with or without TTT (810 nm wavelength at 1, 6, and 12 months after therapy) and followed for a median of 38 months.[31] There were no differences between the two groups in maculopathy, papillopathy, or glaucoma. The enucleation rate was lower in the TTT group (about 2% vs. 18% at 5 years, P = .02). However, the study was not masked, and replication of the results would be important.
There are uncertainties at all stages about the optimal management of intraocular melanoma. Physicians should discuss clinical trial opportunities with eligible patients. Information about ongoing clinical trials is available from the NCI website.
References
- Shields CL, Shields JA, Perez N, et al.: Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology 109 (2): 225-34, 2002. [PUBMED Abstract]
- Augsburger JJ: Is observation really appropriate for small choroidal melanomas. Trans Am Ophthalmol Soc 91: 147-68; discussion 169-75, 1993. [PUBMED Abstract]
- Shields CL, Cater J, Shields JA, et al.: Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol 118 (3): 360-4, 2000. [PUBMED Abstract]
- Robertson DM, Buettner H, Bennett SR: Transpupillary thermotherapy as primary treatment for small choroidal melanomas. Arch Ophthalmol 117 (11): 1512-9, 1999. [PUBMED Abstract]
- Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 115 (12): 1537-44, 1997. [PUBMED Abstract]
- Zimmerman LE, McLean IW, Foster WD: Statistical analysis of follow-up data concerning uveal melanomas, and the influence of enucleation. Ophthalmology 87 (6): 557-64, 1980. [PUBMED Abstract]
- De Potter P, Shields CL, Shields JA: New treatment modalities for uveal melanoma. Curr Opin Ophthalmol 7 (3): 27-32, 1996. [PUBMED Abstract]
- The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: initial mortality findings. COMS report no. 10. Am J Ophthalmol 125 (6): 779-96, 1998. [PUBMED Abstract]
- Hawkins BS; Collaborative Ocular Melanoma Study Group: The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol 138 (6): 936-51, 2004. [PUBMED Abstract]
- Damato B: The role of eyewall resection in uveal melanoma management. Int Ophthalmol Clin 46 (1): 81-93, 2006. [PUBMED Abstract]
- Bechrakis NE, Bornfeld N, Zöller I, et al.: Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 109 (10): 1855-61, 2002. [PUBMED Abstract]
- Bechrakis NE, Petousis V, Willerding G, et al.: Ten-year results of transscleral resection of large uveal melanomas: local tumour control and metastatic rate. Br J Ophthalmol 94 (4): 460-6, 2010. [PUBMED Abstract]
- Hsueh EC, Essner R, Foshag LJ, et al.: Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer 100 (1): 122-9, 2004. [PUBMED Abstract]
- Pawlik TM, Zorzi D, Abdalla EK, et al.: Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol 13 (5): 712-20, 2006. [PUBMED Abstract]
- Albert DM, Kulkarni AD: Intraocular melanoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 2090-8.
- Shields CL, Shields JA, Cater J, et al.: Plaque radiotherapy for uveal melanoma: long-term visual outcome in 1106 consecutive patients. Arch Ophthalmol 118 (9): 1219-28, 2000. [PUBMED Abstract]
- Diener-West M, Earle JD, Fine SL, et al.: The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 119 (7): 969-82, 2001. [PUBMED Abstract]
- Collaborative Ocular Melanoma Study Group: The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 124 (12): 1684-93, 2006. [PUBMED Abstract]
- Melia M, Moy CS, Reynolds SM, et al.: Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3. Arch Ophthalmol 124 (2): 226-38, 2006. [PUBMED Abstract]
- Char DH, Kroll S, Phillips TL, et al.: Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle (proton or helium ion) therapy. Ophthalmology 109 (10): 1850-4, 2002. [PUBMED Abstract]
- Gragoudas E, Li W, Goitein M, et al.: Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol 120 (12): 1665-71, 2002. [PUBMED Abstract]
- Caujolle JP, Mammar H, Chamorey E, et al.: Proton beam radiotherapy for uveal melanomas at nice teaching hospital: 16 years' experience. Int J Radiat Oncol Biol Phys 78 (1): 98-103, 2010. [PUBMED Abstract]
- Tsuji H, Ishikawa H, Yanagi T, et al.: Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a Phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys 67 (3): 857-62, 2007. [PUBMED Abstract]
- Char DH, Quivey JM, Castro JR, et al.: Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology 100 (10): 1547-54, 1993. [PUBMED Abstract]
- Gragoudas ES, Lane AM, Regan S, et al.: A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol 118 (6): 773-8, 2000. [PUBMED Abstract]
- Modorati G, Miserocchi E, Galli L, et al.: Gamma knife radiosurgery for uveal melanoma: 12 years of experience. Br J Ophthalmol 93 (1): 40-4, 2009. [PUBMED Abstract]
- Muller K, Nowak PJ, de Pan C, et al.: Effectiveness of fractionated stereotactic radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 63 (1): 116-22, 2005. [PUBMED Abstract]
- Dieckmann K, Georg D, Bogner J, et al.: Optimizing LINAC-based stereotactic radiotherapy of uveal melanomas: 7 years' clinical experience. Int J Radiat Oncol Biol Phys 66 (4 Suppl 1): 47-52, 2006.
- Harbour JW, Meredith TA, Thompson PA, et al.: Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology 110 (11): 2207-14; discussion 2215, 2003. [PUBMED Abstract]
- Pilotto E, Vujosevic S, De Belvis V, et al.: Long-term choroidal vascular changes after iodine brachytherapy versus transpupillary thermotherapy for choroidal melanoma. Eur J Ophthalmol 19 (4): 646-53, 2009 Jul-Aug. [PUBMED Abstract]
- Desjardins L, Lumbroso-Le Rouic L, Levy-Gabriel C, et al.: Combined proton beam radiotherapy and transpupillary thermotherapy for large uveal melanomas: a randomized study of 151 patients. Ophthalmic Res 38 (5): 255-60, 2006. [PUBMED Abstract]
Treatment of Iris Melanoma
Melanocytic stromal proliferations and nevi of the iris are the most common tumors of the iris, but melanoma is rare.[1,2] Clinical differentiation between an iris nevus and a melanoma might sometimes be difficult and, at times, impossible. Melanomas of the iris are usually small discrete lesions, although they may occasionally be diffuse, infiltrative, or multiple and may result in heterochromia, chronic uveitis, or spontaneous hemorrhage into the anterior chamber of the eye (hyphema). Iris melanomas that involve more than 66% of the angle circumference are associated with secondary glaucoma.[3]
Routine evaluation of iris melanomas includes gonioscopy, transillumination of the globe, and indirect ophthalmoscopy with 360° of scleral depression. Photographic documentation is essential to verify progression in size or growth of the tumor.[4] Anterior segment fluorescein angiography may be helpful to demonstrate the vascularity of the lesion but is not diagnostic. High-resolution ultrasound biomicroscopy can be used to measure small lesions (basal dimensions and thickness) and to assess tumor involvement of the anterior ciliary body, angle, and overlying sclera.[5] The main disadvantage with this technology is its limited penetration of large lesions. In these cases, conventional ultrasonography is more accurate.
In general, patients with iris melanomas have relatively good outcomes. Only about 3% of these melanomas metastasize within 5 years.[1] Iris melanomas are predominantly of the spindle-cell type and are usually smaller in size than posterior melanomas. Clinical features, including prominent tumor vascularity, rapid growth, and heterogeneous pigmentation, are associated with an epithelioid cell component.[6] Involvement of the iridocorneal angles is frequently associated with ciliary body invasion.[6]
Because iris melanomas are rare and have a good prognosis, clinical trials with sufficient power are impractical. Therefore, treatment experience is based principally on case series and case reports. Conservative management is generally advocated whenever possible, but surgical intervention may be justified with unequivocal tumor growth or extensive disease at initial examination.
Treatment Options for Iris Melanoma
Treatment options for iris melanoma include:
- Observation with careful follow-up. This option is used in asymptomatic patients with stable lesions; follow-up includes serial photography.[3]
- Local resection. This option is used when progressive and pronounced growth is documented.[2]
- Enucleation. This option is used if the tumor is not amenable to local resection because of diffuse involvement of the iris, involvement of more than 50% of the iris and anterior chamber angle, intractable glaucoma, or extraocular extension.[7]
- Plaque radiation therapy. This option is offered as an alternative for large, diffuse, surgically nonresectable lesions of the iris.[8]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Albert DM, Kulkarni AD: Intraocular melanoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 2090-8.
- Starr OD, Patel DV, Allen JP, et al.: Iris melanoma: pathology, prognosis and surgical intervention. Clin Experiment Ophthalmol 32 (3): 294-6, 2004. [PUBMED Abstract]
- Marcus DM, Sahel JA, Jakobiec FA, et al.: Pigmented tumors of the iris. In: Albert DM, Jakobiec FA, eds.: Principles and Practice of Ophthalmology. WB Saunders Co., 1994, pp 3198-3208.
- Yap-Veloso MI, Simmons RB, Simmons RJ: Iris melanomas: diagnosis and management. Int Ophthalmol Clin 37 (4): 87-100, 1997 Fall. [PUBMED Abstract]
- Pavlin CJ, McWhae JA, McGowan HD, et al.: Ultrasound biomicroscopy of anterior segment tumors. Ophthalmology 99 (8): 1220-8, 1992. [PUBMED Abstract]
- Conway RM, Chua WC, Qureshi C, et al.: Primary iris melanoma: diagnostic features and outcome of conservative surgical treatment. Br J Ophthalmol 85 (7): 848-54, 2001. [PUBMED Abstract]
- Melanocytic tumors of the iris stroma. In: Shields JA: Diagnosis and Management of Intraocular Tumors. C.V. Mosby Company, 1983, pp 83-121.
- Shields CL, Shields JA, De Potter P, et al.: Treatment of non-resectable malignant iris tumours with custom designed plaque radiotherapy. Br J Ophthalmol 79 (4): 306-12, 1995. [PUBMED Abstract]
Treatment of Ciliary Body Melanoma
Melanoma involving the ciliary body is rare and patients have a poor prognosis. In some cases, diagnosis may be difficult because of similarity to other eye diseases. The differential diagnosis of ciliary body melanoma is considered in cases of unilateral pigmentary glaucoma and chronic uveitis.[1]
Ultrasound biomicroscopy can be used to evaluate tumor shape, thickness, margins, reflectivity, and local invasion.[2,3] Patients with tumors greater than 7 mm in thickness are at increased risk of metastatic disease and melanoma-related death compared with patients with thinner tumors.[4]
Treatment Options for Ciliary Body Melanoma
There are several options for management of ciliary body melanoma. All of them are reported from case series.[Level of evidence C3] The choice of therapy, however, depends on many factors.
Treatment options for ciliary body melanoma include:
- Plaque radiation therapy. Local control rates are high, but treatment is associated with a high incidence of secondary cataract.[4,5]
- External-beam, charged-particle radiation therapy. This approach is offered at specialized referral centers. It requires careful patient cooperation, with voluntary fixation of gaze.[6-8]
- Local tumor resection. This option is mainly suitable for selected ciliary body or anterior choroidal tumors with smaller basal dimension and greater thickness.[9,10]
- Enucleation. This option is generally reserved for large melanomas when regaining useful vision is unlikely. It is also indicated in the presence of intractable secondary glaucoma and extraocular extension.[5,8]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Nguyen QD, Foster CS: Ciliary body melanoma masquerading as chronic uveitis. Ocul Immunol Inflamm 6 (4): 253-6, 1998. [PUBMED Abstract]
- Marigo FA, Finger PT, McCormick SA, et al.: Iris and ciliary body melanomas: ultrasound biomicroscopy with histopathologic correlation. Arch Ophthalmol 118 (11): 1515-21, 2000. [PUBMED Abstract]
- Daftari I, Barash D, Lin S, et al.: Use of high-frequency ultrasound imaging to improve delineation of anterior uveal melanoma for proton irradiation. Phys Med Biol 46 (2): 579-90, 2001. [PUBMED Abstract]
- Gündüz K, Shields CL, Shields JA, et al.: Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol 117 (2): 170-7, 1999. [PUBMED Abstract]
- Finger PT: Plaque radiation therapy for malignant melanoma of the iris and ciliary body. Am J Ophthalmol 132 (3): 328-35, 2001. [PUBMED Abstract]
- Munzenrider JE: Uveal melanomas. Conservation treatment. Hematol Oncol Clin North Am 15 (2): 389-402, 2001. [PUBMED Abstract]
- Char DH, Kroll SM, Castro J: Ten-year follow-up of helium ion therapy for uveal melanoma. Am J Ophthalmol 125 (1): 81-9, 1998. [PUBMED Abstract]
- De Potter P: [Choroidal melanoma: current therapeutic approaches] J Fr Ophtalmol 25 (2): 203-11, 2002. [PUBMED Abstract]
- De Potter P, Shields CL, Shields JA: New treatment modalities for uveal melanoma. Curr Opin Ophthalmol 7 (3): 27-32, 1996. [PUBMED Abstract]
- Char DH, Miller T, Crawford JB: Uveal tumour resection. Br J Ophthalmol 85 (10): 1213-9, 2001. [PUBMED Abstract]
Treatment of Small Choroidal Melanoma
A wide range of 5-year mortality rates has been reported for patients with small choroidal melanomas, with an average rate of about 16%.[1,2] Several studies indicate that the two most important clinical factors predictive of mortality are larger tumor size at the time of treatment and documentation of tumor growth.[3]
The management of small choroidal melanomas is controversial. The likelihood of progression from the time of diagnosis to growth warranting treatment has not been well characterized. Many ophthalmologists advocate initial observation. This initial management strategy is justified on several grounds, including difficulty in establishing a correct diagnosis, lack of documented efficacy for globe-conserving treatments, and concerns for severe treatment-related morbidity. Others have advocated earlier therapeutic intervention.[4-6]
Treatment Options for Small Choroidal Melanoma
Treatment options for small choroidal melanoma include:
- Observation. This strategy is important for patients with an uncertain diagnosis or whose tumor growth has not been documented. It is also used for asymptomatic patients with stable lesions (particularly older or debilitated patients) and for patients with a tumor in their only useful eye.[2]
- Plaque radiation therapy. This treatment is used for small- or medium-sized uveal melanomas, amelanotic tumors, or tumors that touch the optic disc for greater than 3 clock-hours of optic disk circumference.[7,8]
- External-beam, charged-particle radiation therapy. This approach is offered at specialized referral centers. It requires careful patient cooperation, with voluntary fixation of gaze.[7-10]
- Gamma-knife radiation surgery. This treatment may be a feasible option for small- to medium-sized melanomas.[11-13]
- Transpupillary thermotherapy. As noted above, this approach has very limited use, but it can be used as a primary treatment or as an adjunctive method to plaque radiation therapy.[5,6,14-18] For more information, see the Transpupillary Thermotherapy section.
- Local tumor resection. This strategy is used mainly for selected ciliary body or anterior choroidal tumors with smaller basal dimensions and greater thickness.[19]
- Enucleation. This approach is used when severe intraocular pressure elevation is a factor. It may also be considered with small- and medium-sized melanomas that are invading the tissues of the optic nerve.[20]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Diener-West M, Hawkins BS, Markowitz JA, et al.: A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol 110 (2): 245-50, 1992. [PUBMED Abstract]
- Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 115 (7): 886-93, 1997. [PUBMED Abstract]
- Shields CL, Shields JA, Kiratli H, et al.: Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 102 (9): 1351-61, 1995. [PUBMED Abstract]
- Shields CL, Cater J, Shields JA, et al.: Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol 118 (3): 360-4, 2000. [PUBMED Abstract]
- Shields CL, Shields JA, Perez N, et al.: Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology 109 (2): 225-34, 2002. [PUBMED Abstract]
- Robertson DM, Buettner H, Bennett SR: Transpupillary thermotherapy as primary treatment for small choroidal melanomas. Arch Ophthalmol 117 (11): 1512-9, 1999. [PUBMED Abstract]
- Shields CL, Shields JA, Gündüz K, et al.: Radiation therapy for uveal malignant melanoma. Ophthalmic Surg Lasers 29 (5): 397-409, 1998. [PUBMED Abstract]
- Finger PT: Radiation therapy for choroidal melanoma. Surv Ophthalmol 42 (3): 215-32, 1997 Nov-Dec. [PUBMED Abstract]
- Munzenrider JE: Uveal melanomas. Conservation treatment. Hematol Oncol Clin North Am 15 (2): 389-402, 2001. [PUBMED Abstract]
- Char DH, Kroll SM, Castro J: Ten-year follow-up of helium ion therapy for uveal melanoma. Am J Ophthalmol 125 (1): 81-9, 1998. [PUBMED Abstract]
- Woodburn R, Danis R, Timmerman R, et al.: Preliminary experience in the treatment of choroidal melanoma with gamma knife radiosurgery. J Neurosurg 93 (Suppl 3): 177-9, 2000. [PUBMED Abstract]
- Modorati G, Miserocchi E, Galli L, et al.: Gamma knife radiosurgery for uveal melanoma: 12 years of experience. Br J Ophthalmol 93 (1): 40-4, 2009. [PUBMED Abstract]
- Muller K, Nowak PJ, de Pan C, et al.: Effectiveness of fractionated stereotactic radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 63 (1): 116-22, 2005. [PUBMED Abstract]
- Shields CL, Shields JA: Transpupillary thermotherapy for choroidal melanoma. Curr Opin Ophthalmol 10 (3): 197-203, 1999. [PUBMED Abstract]
- Godfrey DG, Waldron RG, Capone A: Transpupillary thermotherapy for small choroidal melanoma. Am J Ophthalmol 128 (1): 88-93, 1999. [PUBMED Abstract]
- Bartlema YM, Oosterhuis JA, Journée-De Korver JG, et al.: Combined plaque radiotherapy and transpupillary thermotherapy in choroidal melanoma: 5 years' experience. Br J Ophthalmol 87 (11): 1370-3, 2003. [PUBMED Abstract]
- Harbour JW, Meredith TA, Thompson PA, et al.: Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology 110 (11): 2207-14; discussion 2215, 2003. [PUBMED Abstract]
- Pilotto E, Vujosevic S, De Belvis V, et al.: Long-term choroidal vascular changes after iodine brachytherapy versus transpupillary thermotherapy for choroidal melanoma. Eur J Ophthalmol 19 (4): 646-53, 2009 Jul-Aug. [PUBMED Abstract]
- Char DH, Miller T, Crawford JB: Uveal tumour resection. Br J Ophthalmol 85 (10): 1213-9, 2001. [PUBMED Abstract]
- Shields JA, Shields CL: Atlas of Intraocular Tumors. Lippincott Williams & Wilkins, 1999.
Treatment of Medium and Large Choroidal Melanoma
Eye-sparing radiation therapy, either by plaque brachytherapy or external beam, is the preferred option for most patients with medium-sized choroidal melanoma. Enucleation remains the standard therapy for large choroidal melanomas and melanomas that cause severe glaucoma or invade the optic nerve.
Treatment Options for Medium and Large Choroidal Melanoma
Tumor growth pattern is a factor in the therapeutic decision. Enucleation is considered for a diffuse melanoma or extraocular extension, but radiation therapy can be employed for less extensive disease.
Treatment options for medium and large choroidal melanoma include:
Medium-sized choroidal melanomas
- Plaque radiation therapy. [1-4]
- External-beam, charged-particle radiation therapy. This approach is offered at specialized referral centers. It requires careful patient cooperation, with voluntary fixation of gaze.[5-7]
- Local eye-wall resection. [8,9]
- Combined therapy, with ablative laser coagulation or transpupillary thermotherapy to supplement plaque treatment.[10,11] For more information, see the Transpupillary Thermotherapy section.
- Enucleation. This approach is considered primarily for diffuse melanomas or extraocular extension. Enucleation may be necessary when there are radiation complications or tumor recurrences.[10]
Large choroidal melanomas
- Enucleation when the tumor is judged to be too large for eye-sparing approaches.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Karvat A, Duzenli C, Ma R, et al.: The treatment of choroidal melanoma with 198 Au plaque brachytherapy. Radiother Oncol 59 (2): 153-6, 2001. [PUBMED Abstract]
- Tabandeh H, Chaudhry NA, Murray TG, et al.: Intraoperative echographic localization of iodine-125 episcleral plaque for brachytherapy of choroidal melanoma. Am J Ophthalmol 129 (2): 199-204, 2000. [PUBMED Abstract]
- Diener-West M, Earle JD, Fine SL, et al.: The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 119 (7): 969-82, 2001. [PUBMED Abstract]
- Melia BM, Abramson DH, Albert DM, et al.: Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology 108 (2): 348-66, 2001. [PUBMED Abstract]
- Char DH, Quivey JM, Castro JR, et al.: Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology 100 (10): 1547-54, 1993. [PUBMED Abstract]
- Fuss M, Loredo LN, Blacharski PA, et al.: Proton radiation therapy for medium and large choroidal melanoma: preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys 49 (4): 1053-9, 2001. [PUBMED Abstract]
- Char DH, Kroll SM, Castro J: Ten-year follow-up of helium ion therapy for uveal melanoma. Am J Ophthalmol 125 (1): 81-9, 1998. [PUBMED Abstract]
- Char DH, Miller T, Crawford JB: Uveal tumour resection. Br J Ophthalmol 85 (10): 1213-9, 2001. [PUBMED Abstract]
- Peyman GA, Juarez CP, Diamond JG, et al.: Ten years experience with eye wall resection for uveal malignant melanomas. Ophthalmology 91 (12): 1720-5, 1984. [PUBMED Abstract]
- Seregard S, Landau I: Transpupillary thermotherapy as an adjunct to ruthenium plaque radiotherapy for choroidal melanoma. Acta Ophthalmol Scand 79 (1): 19-22, 2001. [PUBMED Abstract]
- Shields JA: The expanding role of laser photocoagulation for intraocular tumors. The 1993 H. Christian Zweng Memorial Lecture. Retina 14 (4): 310-22, 1994. [PUBMED Abstract]
Treatment of Extraocular Extension and Metastatic Intraocular Melanoma
Extrascleral extension is associated with a poor prognosis. For patients with gross tumor involvement of the orbit, treatment requires orbital exenteration. However, there is no evidence that such radical surgery will prolong survival. Most patients with localized or encapsulated extraocular extension are not exenterated. This subject is controversial.[1-5]
No effective method of systemic treatment has been identified for patients with metastatic ocular melanoma. Clinical trials are an option for these patients.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Shammas HF, Blodi FC: Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol 95 (1): 63-9, 1977. [PUBMED Abstract]
- Pach JM, Robertson DM, Taney BS, et al.: Prognostic factors in choroidal and ciliary body melanomas with extrascleral extension. Am J Ophthalmol 101 (3): 325-31, 1986. [PUBMED Abstract]
- Kersten RC, Tse DT, Anderson RL, et al.: The role of orbital exenteration in choroidal melanoma with extrascleral extension. Ophthalmology 92 (3): 436-43, 1985. [PUBMED Abstract]
- Hykin PG, McCartney AC, Plowman PN, et al.: Postenucleation orbital radiotherapy for the treatment of malignant melanoma of the choroid with extrascleral extension. Br J Ophthalmol 74 (1): 36-9, 1990. [PUBMED Abstract]
- Gündüz K, Shields CL, Shields JA, et al.: Plaque radiotherapy for management of ciliary body and choroidal melanoma with extraocular extension. Am J Ophthalmol 130 (1): 97-102, 2000. [PUBMED Abstract]
Treatment of Recurrent Intraocular Melanoma
The prognosis for any patient with recurring or relapsing disease is poor, regardless of cell type or stage. The question and selection of further treatment depends on many factors, including the extent of the lesion, age and health of the patient, prior treatment, site of recurrence, and individual patient considerations. Surgical resection of metastases diagnosed after initial management of ocular melanoma in single-center case series of highly selected patients has been reported. The extent to which the occasional favorable outcomes are the result of strong selection factors is not clear, so this approach cannot be considered standard.[1]
Eligible patients should consider participation in clinical trials whenever possible.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hsueh EC, Essner R, Foshag LJ, et al.: Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer 100 (1): 122-9, 2004. [PUBMED Abstract]
Latest Updates to This Summary (05/16/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of intraocular melanoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewer for Intraocular (Uveal) Melanoma Treatment is:
- Shaheer A. Khan, DO (Columbia University Irving Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Intraocular (Uveal) Melanoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/eye/hp/intraocular-melanoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389482]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.