Hodgkin Lymphoma Treatment (PDQ®)–Health Professional Version
General Information About Hodgkin Lymphoma (HL)
Incidence and Mortality
Estimated new cases and deaths from HL in the United States in 2025:[1]
- New cases: 8,720.
- Deaths: 1,150.
Up to 90% of all newly diagnosed patients with HL can be cured with combination chemotherapy and/or radiation therapy.[2]
Anatomy
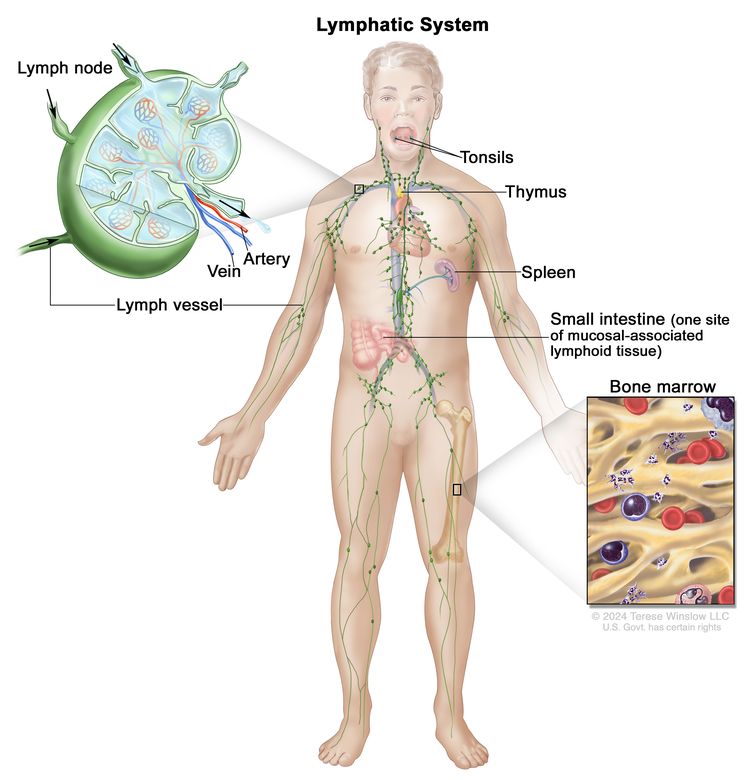
HL most frequently presents in lymph node groups above the diaphragm and/or in mediastinal lymph nodes. Involvement of Waldeyer's ring or tonsillar lymph glands is rarely seen.
Risk Factors
Risk factors for HL include:
- Being in early adulthood (aged 20–39 years) (most often) or late adulthood (aged 65 years and older) (less often).
- Being male.
- Having a previous infection with the Epstein-Barr virus in the teenage years or early childhood.
- Having a first-degree relative with HL.
Clinical Features
These and other signs and symptoms may be caused by HL or by other conditions:
- Painless, swollen lymph nodes in the neck, axilla, or inguinal area.
- Fever defined as 38ºC or higher.
- Drenching and recurrent night sweats.
- Weight loss of 10% or more of baseline weight in the previous 6 months.
- Pruritus, especially after bathing or after ingesting alcohol.
- Fatigue.
Treatment of HL should relieve these symptoms within days. For more information, see Hot Flashes and Night Sweats, Pruritus, and Fatigue.
Diagnostic Evaluation
Diagnostic evaluation of patients with lymphoma may include:
- Biopsy (preferably excisional), with interpretation by a qualified pathologist.
- History, with special attention given to the presence and duration of fever, night sweats, and unexplained weight loss of 10% or more of body weight in the previous 6 months.
- Physical examination.
- Laboratory tests.
- Complete blood cell count and platelet count.
- Erythrocyte sedimentation rate.
- Chemistry panel (electrolytes, blood urea nitrogen, creatinine, calcium, aspartate transaminase, alanine aminotransferase, bilirubin, and alkaline phosphatase) plus lactate dehydrogenase, uric acid, and phosphorus.
- Radiographic examination.
- Computed tomography (CT) of the neck, chest, abdomen, and pelvis; or metabolic imaging (fluorine F 18-fludeoxyglucose positron emission tomography [PET]) with PET-CT. PET-magnetic resonance imaging scans may be equivalent to PET-CT in obtaining staging information at 25% of the radiation dose.[3]
- HIV testing.
- Hepatitis B and hepatitis C serology.
All stages of HL can be subclassified into A and B categories: B for those with defined general symptoms (described below) and A for those without B symptoms. The B designation is given to patients with any of the following symptoms:
- Unexplained weight loss (more than 10% of body weight in the 6 months before diagnosis).
- Unexplained fever with temperatures above 38°C.
- Drenching and recurrent night sweats.
The most significant B symptoms are fevers and weight loss. Night sweats alone do not confer an adverse prognosis.
Prognostic Factors
The prognosis for a given patient depends on several factors. The most important factors include:[1,4,5]
- Presence or absence of systemic B symptoms.
- Stage of disease.
- Presence of large masses.
- Quality and suitability of the treatment administered.
Other important factors are:[1,4,5]
- Age.
- Sex.
- Erythrocyte sedimentation rate.
- Hematocrit.
- Extent of abdominal involvement.
- Absolute number of nodal sites of involvement.
The best predictor of treatment failure is a PET-CT scan obtained after two cycles of chemotherapy (PET2 scan).[6,7] For limited-stage disease, there are frequent false-positive tests because the relapse risk is low (low-positive predictive value). For advanced-stage disease, up to 15% of patients have a relapse despite a negative PET2 scan (lowering the negative predictive value).[6,7] Combining biomarkers with PET-CT scanning responses or calculating metabolic tumor volume with PET-CT scanning are methods under evaluation to improve prognostic predictions.[6,8-11]
Follow-Up
Recommendations for posttreatment follow-up are not evidence based, but a variety of opinions have been published for high-risk patients who present with advanced-stage disease and for patients who achieve less-than-complete remission by PET-CT scans at the end of therapy.[12-15] For patients at high risk of relapse, conventional CT scans are used to avoid increased false-positive test results and increased radiation exposure of serial PET-CT scans.[16]
For patients with negative findings from a PET-CT scan at the end of therapy, routine scans are not advised because of the very low risk of recurrence.[17] Opportunistic scanning is applied when patients present with suspicious symptoms, physical findings, or laboratory test results. The 5-year risk of relapse from diagnosis is 5.6% for patients remaining event-free for 2 years after induction therapy.[18]
Among 6,840 patients enrolled in German Hodgkin Study Group (GHSG) trials, with a median follow-up of 10.3 years, 141 patients had a relapse after 5 years, compared with 466 patients who had a relapse within 5 years. Treatment-related adverse effects and late relapses may occur beyond 20 years of follow-up.[19]
Adverse Long-Term Effects of Therapy
Patients who complete therapy for HL are at risk of developing long-term side effects, ranging from direct damage to organ function or the immune system to second malignancies. For the first 15 years after treatment, HL is the main cause of death. By 15 to 20 years after therapy, the cumulative mortality from a second malignancy, cardiovascular disease, or pulmonary fibrosis exceeds the cumulative mortality from HL.[20-23] This risk of developing a second malignancy is even higher for individuals with a family history of cancer.[24]
Compared with the general population, long-term survivors of HL have a significantly lower life expectancy.[25] A multicenter cohort study of 4,919 patients treated between 1965 and 2000 and before age 51 years had a median follow-up of 20.2 years. Patients with HL had an absolute excess mortality (AEM) of 123 excess deaths per 10,000 person-years. This risk (standardized mortality ratio, 5.2; 95% confidence interval [CI], 4.2–6.5; AEM, 619) was maintained for 40-year survivors.[25] For example, at age 54 years, the cumulative mortality of 20.0% for HL survivors was commensurate with that of a 71-year-old person from the general population. While mortality from HL dropped precipitously from 1965 to 2000, solid tumor mortality did not change over that time.[25]
Second malignancies
Recommendations for screening for secondary malignancies or follow-up of long-term survivors are consensus based and not derived from randomized trials.[26]
Solid tumors
An increase in second solid tumors has also been observed, especially mesothelioma and cancers of the lung, breast, thyroid, bone/soft tissue, stomach, esophagus, colon and rectum, uterine cervix, and head and neck.[27-34] These tumors occur primarily after radiation therapy or with combined-modality treatment (especially when involving mechlorethamine or procarbazine), and approximately 75% occur within radiation ports. The risk of developing a second solid tumor (cumulative incidence of a second cancer) increases with time after treatment.
In a cohort of 18,862 5-year survivors from 13 population-based registries, the younger patients had elevated risks for breast, colon, and rectal cancers for 10 to 25 years before the ages when routine screening is recommended in the general population.[29] Even with involved-field doses of 15 Gy to 25 Gy, sarcomas, breast cancers, and thyroid cancers occurred with similar incidence in young patients, compared with those receiving higher-dose radiation.[35]
Lung cancer and breast cancer are among the most-common second solid tumors that develop after therapy for HL.
- Lung cancer. Lung cancer is seen with increased frequency, even after chemotherapy alone, and the risk of this cancer increases with cigarette smoking.[38-41] In a retrospective Surveillance, Epidemiology, and End Results (SEER) Program analysis, stage-specific survival was decreased by 30% to 60% in HL survivors, compared with patients with de novo non-small cell lung cancer.[42]
- Breast cancer. Breast cancer
is seen with increased frequency after radiation therapy or combined-modality
therapy.[27,28,43-45] The risk appears greatest for females treated with radiation
before age 30 years, especially for girls close to menarche.[46] The incidence of breast cancer increases substantially after 15 years
of posttherapy follow-up.[27,47,48] In a cohort of 1,964 female 5-year HL survivors treated between 1975 and 2008, doxorubicin also increased breast cancer risk independent of age at first treatment or prior chest radiation therapy.[49] Survivors who received more than 200 mg/m2 of doxorubicin had a 1.5-fold increased risk (95% CI, 1.08–2.10) versus survivors who did not receive doxorubicin.
In two case-control studies of 479 patients who developed breast cancer after therapy for HL, cumulative absolute risks for developing breast cancer were calculated as a function of radiation therapy dose and the use of chemotherapy.[50,51] With a 30-year to 40-year follow-up, cumulative absolute risks of breast cancer with exposure to radiation range from 8.5% to 39.6%, depending on age at diagnosis. These cohort studies show a continued increase in cumulative excess risk of breast cancer beyond 20 years of follow-up.[50,51]
In a nested case-control study and subsequent cohort study, patients who received both chemotherapy and radiation therapy had a statistically significant lower risk of developing breast cancer than did those treated with radiation therapy alone.[43,52] Reaching early menopause with fewer than 10 years of intact ovarian function appeared to account for the reduction in risk among patients who received combined-modality therapy.[52] Reduction of radiation volume also decreased the risk of breast cancer after HL.[52]
Late effects of autologous stem cell transplant for failure of induction chemotherapy include second malignancies, hypothyroidism, hypogonadism, herpes zoster, depression, and cardiac disease.[53]
Hematologic cancers
- Acute myelogenous leukemia (AML). Acute nonlymphocytic leukemia may occur in patients treated with combined-modality therapy or with combination chemotherapy alone, especially with increased exposure to alkylating agents.[30,54]
- At 10 years after therapy with regimens containing MOPP (mechlorethamine, vincristine, procarbazine, and prednisone), the risk of AML is approximately 3%, with the peak incidence occurring 5 to 9 years after therapy.[30,54] The risk of acute leukemia at 10 years after therapy with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) appears to be less than 1%.[55]
- A population-based study of more than 35,000 survivors during a 30-year time span identified 217 patients who developed AML. The excess absolute risk (EAR) was significantly higher for older patients (>35 years at diagnosis) than for younger survivors (EAR, 9.9 vs. 4.2 per 10,000 patient-years, P < .001).[56]
Other adverse long-term effects
Treatment of HL also affects the endocrine, cardiac, pulmonary, skeletal, and immune systems. Chronic fatigue can be a debilitating symptom for some long-term survivors.[57] A retrospective survey of 20,007 patients with early- and advanced-stage classical HL treated between 2000 and 2016 (i.e., the era in which ABVD became the preferred frontline chemotherapy regimen) showed 1,321 deaths not attributable to lymphoma (39% of total deaths). Heart disease (estimated EAR: 6.6 per 10,000 patient-years, standardized mortality ratio, 1.7 for early-stage disease and 15.1 per 10,000 patient-years, standardized mortality ratio, 2.1 for advanced-stage disease) and infection (estimated EAR: 3.1 per 10,000 patient-years, standardized mortality ratio, 2.2 for early-stage disease and 10.6 per 10,000 patient-years, standardized mortality ratio, 3.9 for advanced-stage disease) were the leading causes of death, especially in patients older than 60 years.[58]
Infertility. A toxic effect that is primarily related to chemotherapy is infertility, usually after regimens containing MOPP or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone).[59-61] After six to eight cycles of BEACOPP, most men had testosterone levels within reference range; however, 82% of women younger than 30 years recovered menses (mostly within 12 months), but only 45% of women older than 30 years recovered menses.[62] ABVD appears to spare long-term testicular and ovarian function.[60,63,64] Increasing age and alkylator-based regimens are the two major factors increasing the risk of premature ovarian insufficiency.[62,65,66] A prospective evaluation of gonadal function embedded in the randomized Response-Adapted Therapy in Advanced Hodgkin Lymphoma (RATHL) study for patients with newly diagnosed advanced-stage HL found good recovery of anti-Müllerian hormone concentration and reduction in follicle-stimulating hormone after ABVD or AVD (doxorubicin, vinblastine, dacarbazine), but less recovery after BEACOPP and for women older than 35 years.[65] A PET scan-adapted treatment regimen to reduce the use of BEACOPP also resulted in less infertility and gonadal dysfunction.[67] While cryopreservation of oocytes or sperm remains the first choice for preservation of fertility, luteinizing hormone-releasing hormone agonists can be tried in this setting, although efficacy for patients with HL has not been confirmed as has been confirmed for patients with breast cancer.[68] A national Danish registry of 793 HL survivors showed that patients who did not have a relapse had similar parenthood rates to the general population, but assistive reproduction methods were required more often for HL survivors (male, 21.6% vs. 6.3%; female, 13.6% vs. 5.5%; P ≤ .001 for both comparisons).[69]
Hypothyroidism. Hypothyroidism is a late complication primarily related to radiation therapy.[70-72] Long-term survivors who receive radiation therapy to the neck are followed up with annual thyroid-stimulating hormone testing.
Cardiac disease. A late complication primarily related to radiation therapy is cardiac disease, the risk of which may persist for over 30 years after the first treatment.[70,73-81] The EAR of fatal cardiovascular disease ranges from 11.9 to 48.9 per 10,000 patient-years and is mostly attributable to fatal myocardial infarction (MI).[73-75,77] A retrospective survey of over 6,000 patients with HL treated in trials between 1964 and 2004 found that cardiac exposure to radiation and use of doxorubicin were significant predictors of ischemic heart disease, congestive heart failure, arrhythmias, and vascular disease.[79] In a cohort of 7,033 patients with HL, MI mortality risk persisted for 25 years after first treatment with supradiaphragmatic radiation therapy (dependent on the details of treatment planning), doxorubicin, or vincristine.[77,78] A nested case-control study of 2,617 5-year survivors of HL diagnosed before age 51 years and treated between 1965 and 1995 found that the 25-year risk of moderate to severe heart failure increased for patients receiving anthracyclines. The risk ranged from 11.2% for patients exposed to 0 Gy to 15 Gy radiation up to 32.9% for patients exposed to radiation equal or greater than 21 Gy.[82] The use of subcranial blocking did not reduce the incidence of fatal MI in a retrospective review, perhaps because of the exposure of the proximal coronary arteries to radiation.[74] Compared with a general matched population, HL patients treated with mediastinal radiation were at increased risk of complications, especially during cardiac surgery.[83] Risk prediction models rely on the dose of mediastinal radiation, smoking history, male sex, and anthracycline exposure to define the patients at highest risk.[81] These risk prediction models found that mediastinal radiation therapy combined with doxorubicin exposure conferred the highest risk, followed by mediastinal radiation therapy alone.[81]
In the U.K. RAPID trial, performed between 2003 and 2010, 183 patients with early-stage HL were PET-negative but still received involved-field radiation therapy (IFRT) (20 Gy) after receiving ABVD.[80] The average predicted 30-year cardiovascular mortality was 5.02%, which included 3.52% expected in the general population, 0.94% EAR from the doxorubicin, and 0.56% from the IFRT. Since 2010, radiation therapy techniques have advanced by using smaller target volumes, lower-dose IFRT (20 Gy), deep inspiration breath holding, intensity-modulated radiation therapy, and proton beam therapy.[80] These techniques will need further evaluation to better assess cardiovascular risks from radiation therapy.
Pulmonary impairment. Impairment of pulmonary function may occur as a result of mantle-field radiation therapy; this impairment is not usually clinically evident, and recovery in pulmonary testing often occurs after 2 to 3 years.[84] Pulmonary toxic effects from bleomycin as used in ABVD are seen in patients older than 40 years.[85]
Bone necrosis. Avascular necrosis of bone has been observed in patients treated with chemotherapy and is most likely related to corticosteroid therapy.[86]
Bacterial sepsis. Bacterial sepsis may occur rarely after splenectomy performed during staging laparotomy for HL;[87] it is much more common in children than in adults.
Fatigue. Fatigue is a commonly reported symptom among patients who have completed chemotherapy and radiation therapy. In a case-control study design, most HL survivors reported significant fatigue lasting for more than 6 months after therapy, compared with age-matched controls. Quality-of-life questionnaires given to 5,306 patients on GHSG trials showed that 20% of patients complained of severe fatigue 5 years after therapy, and those patients had significantly increased problems with employment and financial stability.[88-90] For more information, see Fatigue.
Neurocognitive impairment. After a median of 23 years from diagnosis, 1,760 HL survivors treated in childhood were compared with 3,180 siblings. Significantly higher rates of memory loss (8.1% vs. 5.7%; P < .05), anxiety (7.0% vs. 5.4%; P < .05), unemployment (9.6% vs. 4.4%; P < .05), depression (9.1% vs. 7.0%; P < .05), and impaired physical quality of life (11.2% vs. 3.0%; P < .05) were reported.[91] Lower risks were associated with survivors who adhered to exercise guidelines and did not smoke, but the design of this study did not allow a cause-and-effect conclusion.
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Brice P, de Kerviler E, Friedberg JW: Classical Hodgkin lymphoma. Lancet 398 (10310): 1518-1527, 2021. [PUBMED Abstract]
- Picardi M, Cavaliere C, Della Pepa R, et al.: PET/MRI for staging patients with Hodgkin lymphoma: equivalent results with PET/CT in a prospective trial. Ann Hematol 100 (6): 1525-1535, 2021. [PUBMED Abstract]
- Cosset JM, Henry-Amar M, Meerwaldt JH, et al.: The EORTC trials for limited stage Hodgkin's disease. The EORTC Lymphoma Cooperative Group. Eur J Cancer 28A (11): 1847-50, 1992. [PUBMED Abstract]
- Evens AM, Helenowski I, Ramsdale E, et al.: A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood 119 (3): 692-5, 2012. [PUBMED Abstract]
- Agostinelli C, Gallamini A, Stracqualursi L, et al.: The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin's lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol 3 (10): e467-e479, 2016. [PUBMED Abstract]
- Gallamini A, Rossi A, Patti C, et al.: Interim PET-adapted chemotherapy in advanced Hodgkin lymphoma: results of the second interim analysis of the Italian GITIL/FIL DH0607 trial. [Abstract] Hematol Oncol 33 (Suppl 1): A-118, 100-180, 2015.
- Spina V, Bruscaggin A, Cuccaro A, et al.: Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood 131 (22): 2413-2425, 2018. [PUBMED Abstract]
- Cottereau AS, Versari A, Loft A, et al.: Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 131 (13): 1456-1463, 2018. [PUBMED Abstract]
- Akhtari M, Milgrom SA, Pinnix CC, et al.: Reclassifying patients with early-stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood 131 (1): 84-94, 2018. [PUBMED Abstract]
- Moskowitz AJ, Schöder H, Gavane S, et al.: Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood 130 (20): 2196-2203, 2017. [PUBMED Abstract]
- Hoppe RT, Advani RH, Ai WZ, et al.: Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw 10 (5): 589-97, 2012. [PUBMED Abstract]
- Ng A, Constine LS, Advani R, et al.: ACR Appropriateness Criteria: follow-up of Hodgkin's lymphoma. Curr Probl Cancer 34 (3): 211-27, 2010 May-Jun. [PUBMED Abstract]
- Armitage JO: Who benefits from surveillance imaging? J Clin Oncol 30 (21): 2579-80, 2012. [PUBMED Abstract]
- Picardi M, Pugliese N, Cirillo M, et al.: Advanced-stage Hodgkin lymphoma: US/chest radiography for detection of relapse in patients in first complete remission--a randomized trial of routine surveillance imaging procedures. Radiology 272 (1): 262-74, 2014. [PUBMED Abstract]
- El-Galaly TC, Mylam KJ, Brown P, et al.: Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica 97 (6): 931-6, 2012. [PUBMED Abstract]
- Hartridge-Lambert SK, Schöder H, Lim RC, et al.: ABVD alone and a PET scan complete remission negates the need for radiologic surveillance in early-stage, nonbulky Hodgkin lymphoma. Cancer 119 (6): 1203-9, 2013. [PUBMED Abstract]
- Hapgood G, Zheng Y, Sehn LH, et al.: Evaluation of the Risk of Relapse in Classical Hodgkin Lymphoma at Event-Free Survival Time Points and Survival Comparison With the General Population in British Columbia. J Clin Oncol 34 (21): 2493-500, 2016. [PUBMED Abstract]
- Bröckelmann PJ, Goergen H, Kohnhorst C, et al.: Late Relapse of Classical Hodgkin Lymphoma: An Analysis of the German Hodgkin Study Group HD7 to HD12 Trials. J Clin Oncol 35 (13): 1444-1450, 2017. [PUBMED Abstract]
- Mauch PM, Kalish LA, Marcus KC, et al.: Long-Term Survival in Hodgkin's Disease Cancer J Sci Am 1 (1): 33-42, 1995. [PUBMED Abstract]
- Aisenberg AC: Problems in Hodgkin's disease management. Blood 93 (3): 761-79, 1999. [PUBMED Abstract]
- Longo DL, Armitage JO: Controversies in the treatment of early-stage Hodgkin's lymphoma. N Engl J Med 372 (17): 1667-9, 2015. [PUBMED Abstract]
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al.: Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 21 (18): 3431-9, 2003. [PUBMED Abstract]
- Sud A, Thomsen H, Sundquist K, et al.: Risk of Second Cancer in Hodgkin Lymphoma Survivors and Influence of Family History. J Clin Oncol 35 (14): 1584-1590, 2017. [PUBMED Abstract]
- de Vries S, Schaapveld M, Janus CPM, et al.: Long-Term Cause-Specific Mortality in Hodgkin Lymphoma Patients. J Natl Cancer Inst 113 (6): 760-769, 2021. [PUBMED Abstract]
- Ng AK: Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood 124 (23): 3373-9, 2014. [PUBMED Abstract]
- Dores GM, Metayer C, Curtis RE, et al.: Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol 20 (16): 3484-94, 2002. [PUBMED Abstract]
- Franklin J, Pluetschow A, Paus M, et al.: Second malignancy risk associated with treatment of Hodgkin's lymphoma: meta-analysis of the randomised trials. Ann Oncol 17 (12): 1749-60, 2006. [PUBMED Abstract]
- Hodgson DC, Gilbert ES, Dores GM, et al.: Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 25 (12): 1489-97, 2007. [PUBMED Abstract]
- Swerdlow AJ, Higgins CD, Smith P, et al.: Second cancer risk after chemotherapy for Hodgkin's lymphoma: a collaborative British cohort study. J Clin Oncol 29 (31): 4096-104, 2011. [PUBMED Abstract]
- Chowdhry AK, McHugh C, Fung C, et al.: Second primary head and neck cancer after Hodgkin lymphoma: a population-based study of 44,879 survivors of Hodgkin lymphoma. Cancer 121 (9): 1436-45, 2015. [PUBMED Abstract]
- Dores GM, Curtis RE, van Leeuwen FE, et al.: Pancreatic cancer risk after treatment of Hodgkin lymphoma. Ann Oncol 25 (10): 2073-9, 2014. [PUBMED Abstract]
- Rigter LS, Spaander MCW, Aleman BMP, et al.: High prevalence of advanced colorectal neoplasia and serrated polyposis syndrome in Hodgkin lymphoma survivors. Cancer 125 (6): 990-999, 2019. [PUBMED Abstract]
- Geurts YM, Shakir R, Ntentas G, et al.: Association of Radiation and Procarbazine Dose With Risk of Colorectal Cancer Among Survivors of Hodgkin Lymphoma. JAMA Oncol 9 (4): 481-489, 2023. [PUBMED Abstract]
- O'Brien MM, Donaldson SS, Balise RR, et al.: Second malignant neoplasms in survivors of pediatric Hodgkin's lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol 28 (7): 1232-9, 2010. [PUBMED Abstract]
- Bonadonna G, Viviani S, Bonfante V, et al.: Survival in Hodgkin's disease patients--report of 25 years of experience at the Milan Cancer Institute. Eur J Cancer 41 (7): 998-1006, 2005. [PUBMED Abstract]
- Schaapveld M, Aleman BM, van Eggermond AM, et al.: Second Cancer Risk Up to 40 Years after Treatment for Hodgkin's Lymphoma. N Engl J Med 373 (26): 2499-511, 2015. [PUBMED Abstract]
- van Leeuwen FE, Klokman WJ, Stovall M, et al.: Roles of radiotherapy and smoking in lung cancer following Hodgkin's disease. J Natl Cancer Inst 87 (20): 1530-7, 1995. [PUBMED Abstract]
- Swerdlow AJ, Schoemaker MJ, Allerton R, et al.: Lung cancer after Hodgkin's disease: a nested case-control study of the relation to treatment. J Clin Oncol 19 (6): 1610-8, 2001. [PUBMED Abstract]
- Travis LB, Gospodarowicz M, Curtis RE, et al.: Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst 94 (3): 182-92, 2002. [PUBMED Abstract]
- Lorigan P, Radford J, Howell A, et al.: Lung cancer after treatment for Hodgkin's lymphoma: a systematic review. Lancet Oncol 6 (10): 773-9, 2005. [PUBMED Abstract]
- Milano MT, Li H, Constine LS, et al.: Survival after second primary lung cancer: a population-based study of 187 Hodgkin lymphoma patients. Cancer 117 (24): 5538-47, 2011. [PUBMED Abstract]
- van Leeuwen FE, Klokman WJ, Stovall M, et al.: Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst 95 (13): 971-80, 2003. [PUBMED Abstract]
- Wahner-Roedler DL, Nelson DF, Croghan IT, et al.: Risk of breast cancer and breast cancer characteristics in women treated with supradiaphragmatic radiation for Hodgkin lymphoma: Mayo Clinic experience. Mayo Clin Proc 78 (6): 708-15, 2003. [PUBMED Abstract]
- Travis LB, Hill DA, Dores GM, et al.: Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 290 (4): 465-75, 2003. [PUBMED Abstract]
- Moskowitz CS, Ronckers CM, Chou JF, et al.: Development and Validation of a Breast Cancer Risk Prediction Model for Childhood Cancer Survivors Treated With Chest Radiation: A Report From the Childhood Cancer Survivor Study and the Dutch Hodgkin Late Effects and LATER Cohorts. J Clin Oncol 39 (27): 3012-3021, 2021. [PUBMED Abstract]
- Alm El-Din MA, Hughes KS, Finkelstein DM, et al.: Breast cancer after treatment of Hodgkin's lymphoma: risk factors that really matter. Int J Radiat Oncol Biol Phys 73 (1): 69-74, 2009. [PUBMED Abstract]
- Cooke R, Jones ME, Cunningham D, et al.: Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br J Cancer 108 (11): 2399-406, 2013. [PUBMED Abstract]
- Neppelenbroek SIM, Geurts YM, Aleman BMP, et al.: Doxorubicin Exposure and Breast Cancer Risk in Survivors of Adolescent and Adult Hodgkin Lymphoma. J Clin Oncol 42 (16): 1903-1913, 2024. [PUBMED Abstract]
- Travis LB, Hill D, Dores GM, et al.: Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst 97 (19): 1428-37, 2005. [PUBMED Abstract]
- Swerdlow AJ, Cooke R, Bates A, et al.: Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin's lymphoma in England and Wales: a National Cohort Study. J Clin Oncol 30 (22): 2745-52, 2012. [PUBMED Abstract]
- De Bruin ML, Sparidans J, van't Veer MB, et al.: Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27 (26): 4239-46, 2009. [PUBMED Abstract]
- Lavoie JC, Connors JM, Phillips GL, et al.: High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood 106 (4): 1473-8, 2005. [PUBMED Abstract]
- Koontz MZ, Horning SJ, Balise R, et al.: Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol 31 (5): 592-8, 2013. [PUBMED Abstract]
- Valagussa P, Santoro A, Fossati-Bellani F, et al.: Second acute leukemia and other malignancies following treatment for Hodgkin's disease. J Clin Oncol 4 (6): 830-7, 1986. [PUBMED Abstract]
- Schonfeld SJ, Gilbert ES, Dores GM, et al.: Acute myeloid leukemia following Hodgkin lymphoma: a population-based study of 35,511 patients. J Natl Cancer Inst 98 (3): 215-8, 2006. [PUBMED Abstract]
- Kreissl S, Müller H, Goergen H, et al.: Health-Related Quality of Life in Patients With Hodgkin Lymphoma: A Longitudinal Analysis of the German Hodgkin Study Group. J Clin Oncol 38 (25): 2839-2848, 2020. [PUBMED Abstract]
- Dores GM, Curtis RE, Dalal NH, et al.: Cause-Specific Mortality Following Initial Chemotherapy in a Population-Based Cohort of Patients With Classical Hodgkin Lymphoma, 2000-2016. J Clin Oncol 38 (35): 4149-4162, 2020. [PUBMED Abstract]
- Behringer K, Breuer K, Reineke T, et al.: Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol 23 (30): 7555-64, 2005. [PUBMED Abstract]
- van der Kaaij MA, Heutte N, Le Stang N, et al.: Gonadal function in males after chemotherapy for early-stage Hodgkin's lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 25 (19): 2825-32, 2007. [PUBMED Abstract]
- Scholz M, Engert A, Franklin J, et al.: Impact of first- and second-line treatment for Hodgkin's lymphoma on the incidence of AML/MDS and NHL--experience of the German Hodgkin's Lymphoma Study Group analyzed by a parametric model of carcinogenesis. Ann Oncol 22 (3): 681-8, 2011. [PUBMED Abstract]
- Behringer K, Mueller H, Goergen H, et al.: Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol 31 (2): 231-9, 2013. [PUBMED Abstract]
- Viviani S, Santoro A, Ragni G, et al.: Pre- and post-treatment testicular dysfunction in Hodgkin's disease (HD). [Abstract] Proceedings of the American Society of Clinical Oncology 7: A-877, 227, 1988.
- van der Kaaij MA, Heutte N, Meijnders P, et al.: Premature ovarian failure and fertility in long-term survivors of Hodgkin's lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol 30 (3): 291-9, 2012. [PUBMED Abstract]
- Anderson RA, Remedios R, Kirkwood AA, et al.: Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin's lymphoma (RATHL): a secondary analysis of a randomised phase 3 trial. Lancet Oncol 19 (10): 1328-1337, 2018. [PUBMED Abstract]
- Weibull CE, Johansson ALV, Eloranta S, et al.: Contemporarily Treated Patients With Hodgkin Lymphoma Have Childbearing Potential in Line With Matched Comparators. J Clin Oncol 36 (26): 2718-2725, 2018. [PUBMED Abstract]
- Demeestere I, Racape J, Dechene J, et al.: Gonadal Function Recovery in Patients With Advanced Hodgkin Lymphoma Treated With a PET-Adapted Regimen: Prospective Analysis of a Randomized Phase III Trial (AHL2011). J Clin Oncol 39 (29): 3251-3260, 2021. [PUBMED Abstract]
- Lambertini M, Demeestere I: Another step towards improving oncofertility counselling of young women with Hodgkin's lymphoma. Lancet Oncol 19 (10): 1264-1266, 2018. [PUBMED Abstract]
- Øvlisen AK, Jakobsen LH, Eloranta S, et al.: Parenthood Rates and Use of Assisted Reproductive Techniques in Younger Hodgkin Lymphoma Survivors: A Danish Population-Based Study. J Clin Oncol 39 (31): 3463-3472, 2021. [PUBMED Abstract]
- Tarbell NJ, Thompson L, Mauch P: Thoracic irradiation in Hodgkin's disease: disease control and long-term complications. Int J Radiat Oncol Biol Phys 18 (2): 275-81, 1990. [PUBMED Abstract]
- Hancock SL, Cox RS, McDougall IR: Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med 325 (9): 599-605, 1991. [PUBMED Abstract]
- Cella L, Conson M, Caterino M, et al.: Thyroid V30 predicts radiation-induced hypothyroidism in patients treated with sequential chemo-radiotherapy for Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys 82 (5): 1802-8, 2012. [PUBMED Abstract]
- Reinders JG, Heijmen BJ, Olofsen-van Acht MJ, et al.: Ischemic heart disease after mantlefield irradiation for Hodgkin's disease in long-term follow-up. Radiother Oncol 51 (1): 35-42, 1999. [PUBMED Abstract]
- Hancock SL, Tucker MA, Hoppe RT: Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 270 (16): 1949-55, 1993. [PUBMED Abstract]
- Heidenreich PA, Schnittger I, Strauss HW, et al.: Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol 25 (1): 43-9, 2007. [PUBMED Abstract]
- Dabaja B, Cox JD, Buchholz TA: Radiation therapy can still be used safely in combined modality approaches in patients with Hodgkin's lymphoma. J Clin Oncol 25 (1): 3-5, 2007. [PUBMED Abstract]
- Swerdlow AJ, Higgins CD, Smith P, et al.: Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 99 (3): 206-14, 2007. [PUBMED Abstract]
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al.: Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol 34 (3): 235-43, 2016. [PUBMED Abstract]
- Maraldo MV, Giusti F, Vogelius IR, et al.: Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2 (11): e492-502, 2015. [PUBMED Abstract]
- Cutter DJ, Ramroth J, Diez P, et al.: Predicted Risks of Cardiovascular Disease Following Chemotherapy and Radiotherapy in the UK NCRI RAPID Trial of Positron Emission Tomography-Directed Therapy for Early-Stage Hodgkin Lymphoma. J Clin Oncol 39 (32): 3591-3601, 2021. [PUBMED Abstract]
- de Vries S, Haaksma ML, Jóźwiak K, et al.: Development and Validation of Risk Prediction Models for Coronary Heart Disease and Heart Failure After Treatment for Hodgkin Lymphoma. J Clin Oncol 41 (1): 86-95, 2023. [PUBMED Abstract]
- van Nimwegen FA, Ntentas G, Darby SC, et al.: Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 129 (16): 2257-2265, 2017. [PUBMED Abstract]
- Galper SL, Yu JB, Mauch PM, et al.: Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 117 (2): 412-8, 2011. [PUBMED Abstract]
- Horning SJ, Adhikari A, Rizk N, et al.: Effect of treatment for Hodgkin's disease on pulmonary function: results of a prospective study. J Clin Oncol 12 (2): 297-305, 1994. [PUBMED Abstract]
- Martin WG, Ristow KM, Habermann TM, et al.: Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol 23 (30): 7614-20, 2005. [PUBMED Abstract]
- Prosnitz LR, Lawson JP, Friedlaender GE, et al.: Avascular necrosis of bone in Hodgkin's disease patients treated with combined modality therapy. Cancer 47 (12): 2793-7, 1981. [PUBMED Abstract]
- Schimpff SC, O'Connell MJ, Greene WH, et al.: Infections in 92 splenectomized patients with Hodgkin's disease. A clinical review. Am J Med 59 (5): 695-701, 1975. [PUBMED Abstract]
- Behringer K, Goergen H, Müller H, et al.: Cancer-Related Fatigue in Patients With and Survivors of Hodgkin Lymphoma: The Impact on Treatment Outcome and Social Reintegration. J Clin Oncol 34 (36): 4329-4337, 2016. [PUBMED Abstract]
- Loge JH, Abrahamsen AF, Ekeberg O, et al.: Hodgkin's disease survivors more fatigued than the general population. J Clin Oncol 17 (1): 253-61, 1999. [PUBMED Abstract]
- Kreissl S, Mueller H, Goergen H, et al.: Cancer-related fatigue in patients with and survivors of Hodgkin's lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol 17 (10): 1453-1462, 2016. [PUBMED Abstract]
- Williams AM, Mirzaei Salehabadi S, Xing M, et al.: Modifiable risk factors for neurocognitive and psychosocial problems after Hodgkin lymphoma. Blood 139 (20): 3073-3086, 2022. [PUBMED Abstract]
Cellular Classification of HL
Pathologists currently use the World Health Organization (WHO) modification of the Revised European-American Lymphoma (REAL) classification for the histological classification of Hodgkin lymphoma (HL).[1,2]
WHO Modification of the REAL Classification
- Classic HL.
- Nodular sclerosis HL.
- Mixed-cellularity HL.
- Lymphocyte-depleted HL. Among 10,019 patients who underwent central expert pathology review for the German Hodgkin Study Group, 84 patients (<1%) were identified as having lymphocyte-depleted classic HL.[3] These patients presented more frequently with advanced-stage HL and B symptoms.
- Lymphocyte-rich classic HL.
- Nodular lymphocyte–predominant HL (NLPHL). NLPHL is a clinicopathological entity of B-cell origin that is distinct from classic HL.[4,5]
The typical immunophenotype for classic HL is CD15+, CD20-, CD30+, CD45-, while the profile for lymphocyte-predominant disease is CD15-, CD20+, CD30-, CD45+.
References
- Lukes RJ, Craver LF, Hall TC, et al.: Report of the Nomenclature Committee. Cancer Res 26 (1): 1311, 1966.
- Harris NL: Hodgkin's lymphomas: classification, diagnosis, and grading. Semin Hematol 36 (3): 220-32, 1999. [PUBMED Abstract]
- Klimm B, Franklin J, Stein H, et al.: Lymphocyte-depleted classical Hodgkin's lymphoma: a comprehensive analysis from the German Hodgkin study group. J Clin Oncol 29 (29): 3914-20, 2011. [PUBMED Abstract]
- Eichenauer DA, Plütschow A, Fuchs M, et al.: Long-Term Follow-Up of Patients With Nodular Lymphocyte-Predominant Hodgkin Lymphoma Treated in the HD7 to HD15 Trials: A Report From the German Hodgkin Study Group. J Clin Oncol 38 (7): 698-705, 2020. [PUBMED Abstract]
- Bartlett NL: Treatment of Nodular Lymphocyte Hodgkin Lymphoma: The Goldilocks Principle. J Clin Oncol 38 (7): 662-668, 2020. [PUBMED Abstract]
Stage Information for HL
Clinical staging for patients with Hodgkin lymphoma (HL) includes:
Staging laparotomy is no longer recommended and should be considered only when the results will allow substantially less treatment. Staging laparotomy should not be done in patients who require chemotherapy. If the laparotomy is required for treatment decisions, the risks of potential morbidity should be considered.[3-6]
Bone marrow involvement occurs in 5% of patients and is more prevalent in the context of constitutional B symptoms and anemia, leukopenia, or thrombocytopenia. In a retrospective review and meta-analysis of 955 patients in nine studies, fewer than 2% of patients with positive bone marrow biopsy results had only stage I or stage II disease on PET-CT scans.[7] Omission of the bone marrow biopsy for PET-CT–designated early-stage patients did not change treatment selection.[7] In addition, focal skeletal bone lesions on PET-CT predicted bone marrow involvement with a 96.9% (95% confidence interval [CI], 93.0%–99.08%) sensitivity and 99.7% (95% CI, 98.9%–100%) specificity.[7] For these reasons, PET-CT has replaced bone marrow biopsy in the clinical staging of newly diagnosed HL.
Massive mediastinal disease has been defined by the Cotswolds meeting as a thoracic ratio of maximum transverse mass diameter of 33% or more of the internal transverse thoracic diameter measured at the T5/6 intervertebral disc level on chest radiography.[1] Some investigators have designated a lymph node mass measuring 10 cm or more in greatest dimension as massive disease.[8] Other investigators use a measurement of the maximum width of the mediastinal mass divided by the maximum intrathoracic diameter.[9]
Staging Subclassification System
Lugano Classification
The American Joint Committee on Cancer (AJCC) has adopted the Lugano classification to evaluate and stage lymphoma.[10] The Lugano classification system replaces the Ann Arbor classification system, which was adopted in 1971 at the Ann Arbor Conference,[11] with some modifications 18 years later from the Cotswolds meeting.[1]
| Stage | Stage Description | Illustration |
|---|---|---|
| CSF = cerebrospinal fluid; CT = computed tomography; DLBCL = diffuse large B-cell lymphoma; NHL = non-Hodgkin lymphoma. | ||
| aHodgkin and Non-Hodgkin Lymphomas. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 937–58. | ||
| bStage II bulky may be considered either early or advanced stage based on lymphoma histology and prognostic factors. | ||
| cThe definition of disease bulk varies according to lymphoma histology. In the Lugano classification, bulk ln Hodgkin lymphoma is defined as a mass greater than one-third of the thoracic diameter on CT of the chest or a mass >10 cm. For NHL, the recommended definitions of bulk vary by lymphoma histology. In follicular lymphoma, 6 cm has been suggested based on the Follicular Lymphoma International Prognostic Index-2 and its validation. In DLBCL, cutoffs ranging from 5 cm to 10 cm have been used, although 10 cm is recommended. | ||
| Limited stage | ||
| I | Involvement of a single lymphatic site (i.e., nodal region, Waldeyer’s ring, thymus, or spleen). |
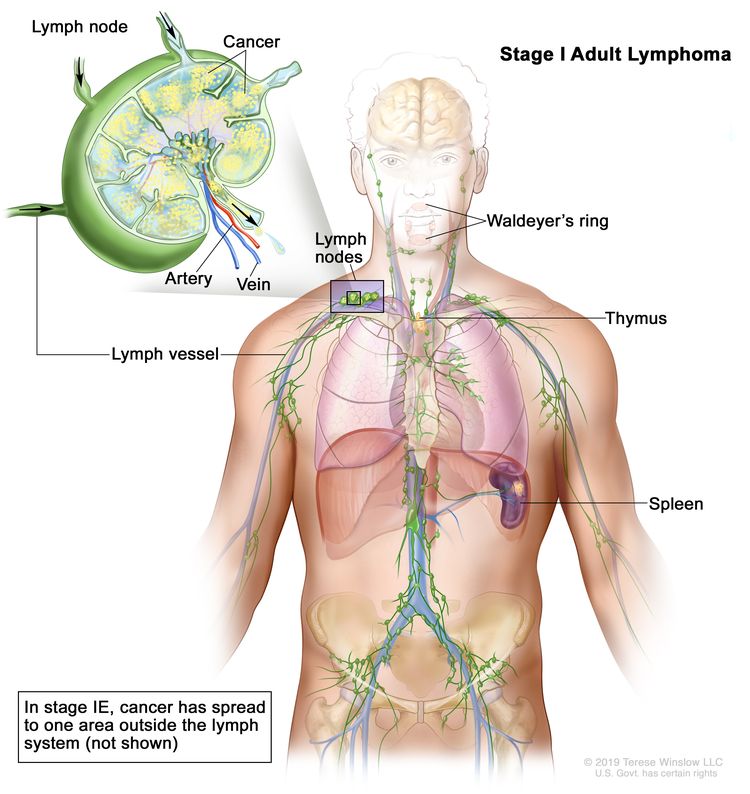 |
| IE | Single extralymphatic site in the absence of nodal involvement (rare in Hodgkin lymphoma). | |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm. |
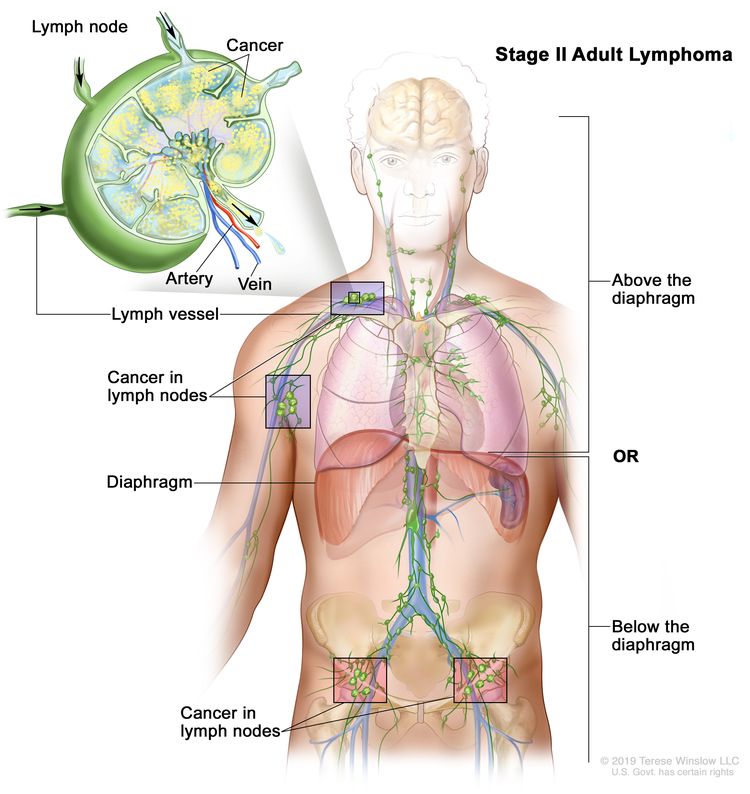 |
| IIE | Contiguous extralymphatic extension from a nodal site with or without involvement of other lymph node regions on the same side of the diaphragm. |
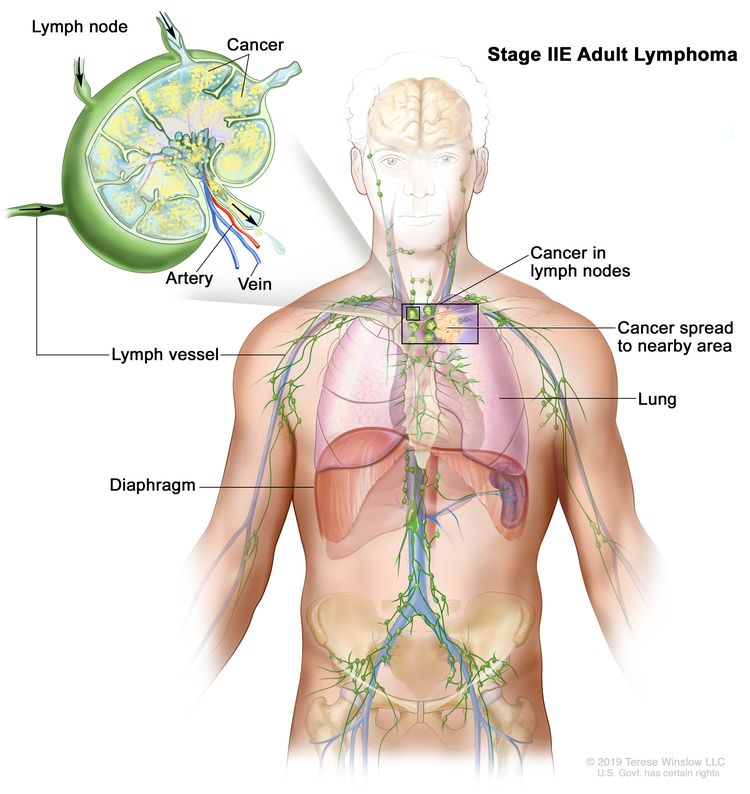 |
| II bulkyb | Stage II with disease bulk.c | |
| Advanced stage | ||
| III | Involvement of lymph node regions on both sides of the diaphragm; nodes above the diaphragm with spleen involvement. |
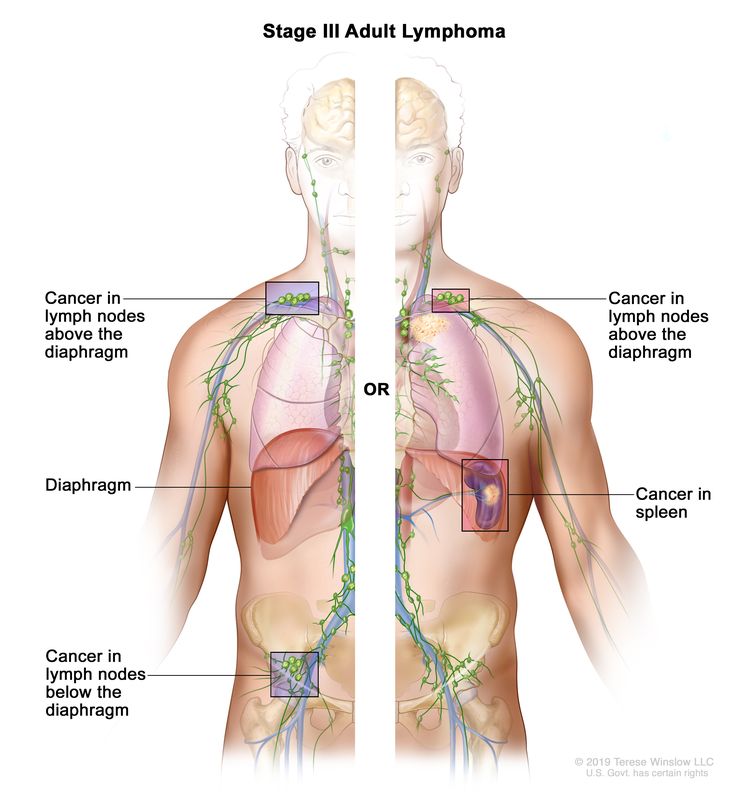 |
| IV | Diffuse or disseminated involvement of one or more extralymphatic organs, with or without associated lymph node involvement; or noncontiguous extralymphatic organ involvement in conjunction with nodal stage II disease; or any extralymphatic organ involvement in nodal stage III disease. Stage IV includes any involvement of the CSF, bone marrow, liver, or multiple lung lesions (other than by direct extension in stage IIE disease). |
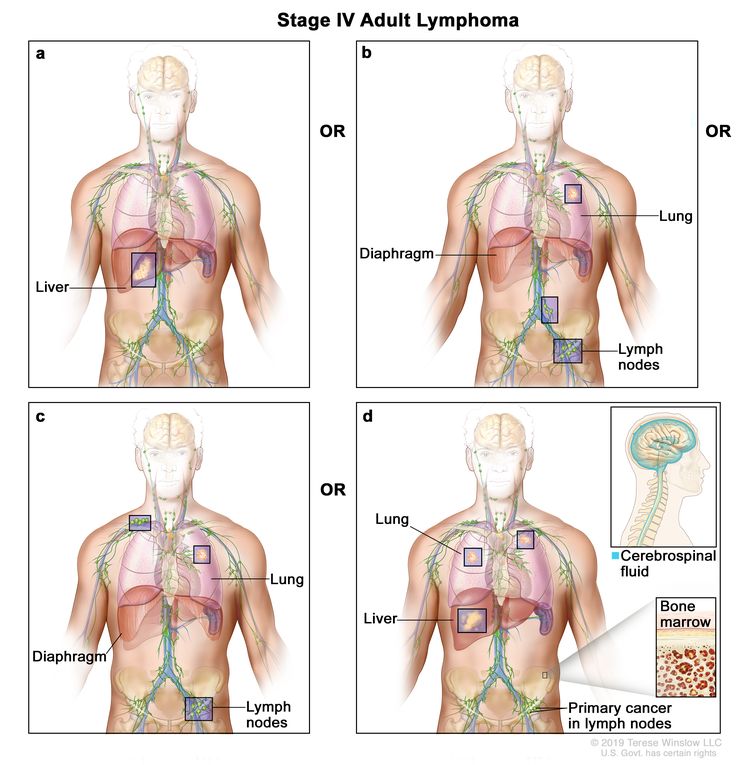 |
| Note: Hodgkin lymphoma uses A or B designation with stage group. A/B is no longer used in NHL. | ||
The E designation is used when well-localized extranodal lymphoid malignancies arise in or extend to tissues beyond, but near, the major lymphatic aggregates. Stage IV refers to disease that is diffusely spread throughout an extranodal site, such as the liver. If pathological proof of involvement of one or more extralymphatic sites has been documented, the symbol for the site of involvement, followed by a plus sign (+), is listed.
| N = nodes | H = liver | L = lung | M = bone marrow |
| S = spleen | P = pleura | O = bone | D = skin |
Prognostic Groups
Many investigators and many new clinical trials employ a clinical staging system that divides patients into three major groups that are also useful for the clinician:[12]
- Early favorable.
- Early unfavorable.
- Advanced.
The group assignment depends on:
- Whether the patient has early or advanced disease.
- The type and number of adverse prognostic factors present.
Early-stage adverse prognostic factors:
- Large mediastinal mass (>33% of the thoracic width on chest x-ray, ≥10 cm on CT scan).
- Extranodal involvement.
- Elevated erythrocyte sedimentation rate (>30 mm/h for B stage [symptoms], >50 mm/h for A stage [symptoms]).
- Involvement of three or more lymph node areas.
- Presence of B symptoms.
Early favorable group: Clinical stage I or II without any of the adverse prognostic factors listed above.
Early unfavorable group: Clinical stage I or II with one or more of the adverse prognostic factors listed above.
Advanced-stage adverse prognostic factors:
For patients with advanced-stage HL, the International Prognostic Factors Project on Advanced Hodgkin's Disease developed the International Prognostic Index with a score that is based on the following seven adverse prognostic factors:[13]
- Albumin level lower than 40 g/L.
- Hemoglobin level lower than 105 g/L.
- Male sex.
- Age 45 years or older.
- Stage IV disease.
- White blood cell (WBC) count of 15 × 109/L or higher.
- Absolute lymphocytic count lower than 0.6 × 109/L or lymphocyte count higher than 8% of the total WBC count.
Advanced group: Clinical stage III or IV with up to three of the adverse risk factors listed above. Patients with advanced disease have a 60% to 80% rate of freedom from progression of disease at 5 years from treatment with first-line chemotherapy.[13][Level of evidence C2] An updated clinical prediction model uses continuous variables listed for the International Prognostic Index above, with an online calculator available.[14]
References
- Lister TA, Crowther D, Sutcliffe SB, et al.: Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7 (11): 1630-6, 1989. [PUBMED Abstract]
- Barrington SF, Kirkwood AA, Franceschetto A, et al.: PET-CT for staging and early response: results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood 127 (12): 1531-8, 2016. [PUBMED Abstract]
- Urba WJ, Longo DL: Hodgkin's disease. N Engl J Med 326 (10): 678-87, 1992. [PUBMED Abstract]
- Sombeck MD, Mendenhall NP, Kaude JV, et al.: Correlation of lymphangiography, computed tomography, and laparotomy in the staging of Hodgkin's disease. Int J Radiat Oncol Biol Phys 25 (3): 425-9, 1993. [PUBMED Abstract]
- Mauch P, Larson D, Osteen R, et al.: Prognostic factors for positive surgical staging in patients with Hodgkin's disease. J Clin Oncol 8 (2): 257-65, 1990. [PUBMED Abstract]
- Dietrich PY, Henry-Amar M, Cosset JM, et al.: Second primary cancers in patients continuously disease-free from Hodgkin's disease: a protective role for the spleen? Blood 84 (4): 1209-15, 1994. [PUBMED Abstract]
- Adams HJ, Kwee TC, de Keizer B, et al.: Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol 25 (5): 921-7, 2014. [PUBMED Abstract]
- Bradley AJ, Carrington BM, Lawrance JA, et al.: Assessment and significance of mediastinal bulk in Hodgkin's disease: comparison between computed tomography and chest radiography. J Clin Oncol 17 (8): 2493-8, 1999. [PUBMED Abstract]
- Mauch P, Goodman R, Hellman S: The significance of mediastinal involvement in early stage Hodgkin's disease. Cancer 42 (3): 1039-45, 1978. [PUBMED Abstract]
- Hodgkin and non-Hodgkin lymphoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 937–58.
- Carbone PP, Kaplan HS, Musshoff K, et al.: Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31 (11): 1860-1, 1971. [PUBMED Abstract]
- Jost LM, Stahel RA; ESMO Guidelines Task Force: ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of Hodgkin's disease. Ann Oncol 16 (Suppl 1): i54-5, 2005. [PUBMED Abstract]
- Hasenclever D, Diehl V: A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med 339 (21): 1506-14, 1998. [PUBMED Abstract]
- Rodday AM, Parsons SK, Upshaw JN, et al.: The Advanced-Stage Hodgkin Lymphoma International Prognostic Index: Development and Validation of a Clinical Prediction Model From the HoLISTIC Consortium. J Clin Oncol 41 (11): 2076-2086, 2023. [PUBMED Abstract]
Treatment Option Overview for HL
After initial clinical staging for Hodgkin lymphoma (HL), patients with early favorable disease or early unfavorable disease are treated with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy with or without involved-field or nodal radiation.
Patients with advanced-stage disease are primarily treated with chemotherapy alone, although subsequent radiation therapy may be applied for initial bulky disease (≥10 cm mediastinal mass) or for residual adenopathy (>2.5 cm) with positive findings after a postchemotherapy positron emission tomography (PET) scan.[1] Treatment regimen preferences and application, as well as relative risks, differ regionally.
Patients with HL who are older than 60 years may have more treatment-related morbidity and mortality; maintaining the dose intensity of standard chemotherapy may be difficult.[2,3] Other therapies have been proposed for older patients with lower tolerance for conventional regimens, but no randomized trials have been conducted with these regimens.[4] Twenty-seven previously untreated patients older than 60 years, judged by the investigator to be in poor condition and unable to undergo chemotherapy, received brentuximab vedotin. A 92% overall response rate and 73% complete remission rate were reported.[5][Level of evidence C3] Brentuximab vedotin has been combined with dacarbazine [6] or sequentially with AVD (doxorubicin, vinblastine, dacarbazine) [7], reporting acceptable toxicities in an older population. A retrospective review of 287 patients aged 60 years or older with early-stage favorable HL in two German Hodgkin Study Group (GHSG) trials (HD10 and HD13) showed increased bleomycin-induced lung toxicity with more than two cycles of exposure to bleomycin.[8]
| Prognostic Group | Treatment Options |
|---|---|
| HL = Hodgkin lymphoma; NLPHL = nodular lymphocyte-predominant Hodgkin lymphoma. | |
| Early favorable classic HL | Chemotherapy with or without radiation therapy |
| Early unfavorable classic HL | Chemotherapy with or without radiation therapy |
| Advanced classic HL | Chemotherapy with or without nivolumab or brentuximab vedotin |
| Recurrent classic HL | Pembrolizumab or nivolumab (alone or with chemotherapy) |
| Brentuximab vedotin | |
| Brentuximab vedotin plus nivolumab | |
| Chemotherapy with stem cell transplant | |
| Combination chemotherapy | |
| Radiation therapy | |
| NLPHL | Watchful waiting/active surveillance |
| Radiation therapy | |
| Chemotherapy | |
| Rituximab | |
Chemotherapy
Table 4 describes the chemotherapy regimens used in the treatment of HL.
| Combination Name | Drugs Included | Prognostic Group |
|---|---|---|
| HL = Hodgkin lymphoma. | ||
| ABVD | Doxorubicin, bleomycin, vinblastine, and dacarbazine | Early favorable classic HL |
| Early unfavorable classic HL | ||
| Advanced classic HL | ||
| BEACOPP | Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone | Early unfavorable classic HL |
| Advanced classic HL | ||
| GVD | Gemcitabine, vinorelbine, and liposomal doxorubicin | Recurrent classic HL |
| ICE | Ifosfamide, carboplatin, and etoposide | Recurrent classic HL |
| MOPP | Mechlorethamine, vincristine, procarbazine, and prednisone | Advanced classic HL |
Radiation Therapy
Radiation therapy alone is almost never used to treat patients newly diagnosed with early favorable classic HL.[9] In HL, the appropriate dose of radiation alone is 20 Gy to 30 Gy to clinically uninvolved sites and 30 Gy to 36 Gy to regions of initial nodal involvement.[9-11] When mediastinal radiation will encompass the left side of the heart or will increase breast cancer risk in young female patients, proton therapy may be considered to reduce the radiation dose to organs at risk.[12] When used as a single modality, radiation therapy is delivered to the neck, chest, and axilla (mantle field) and then to an abdominal field to treat para-aortic nodes and the spleen (splenic pedicle). In some patients, pelvic nodes are treated with a third field. The three fields constitute total nodal radiation therapy. In some cases, the pelvic and para-aortic nodes are treated in a single field called an inverted Y.[9-11]
References
- Engert A, Haverkamp H, Kobe C, et al.: Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet 379 (9828): 1791-9, 2012. [PUBMED Abstract]
- Böll B, Görgen H, Fuchs M, et al.: ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol 31 (12): 1522-9, 2013. [PUBMED Abstract]
- Evens AM, Hong F: How can outcomes be improved for older patients with Hodgkin lymphoma? J Clin Oncol 31 (12): 1502-5, 2013. [PUBMED Abstract]
- Kolstad A, Nome O, Delabie J, et al.: Standard CHOP-21 as first line therapy for elderly patients with Hodgkin's lymphoma. Leuk Lymphoma 48 (3): 570-6, 2007. [PUBMED Abstract]
- Forero-Torres A, Holkova B, Goldschmidt J, et al.: Phase 2 study of frontline brentuximab vedotin monotherapy in Hodgkin lymphoma patients aged 60 years and older. Blood 126 (26): 2798-804, 2015. [PUBMED Abstract]
- Friedberg JW, Forero-Torres A, Bordoni RE, et al.: Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged ≥60 years with HL. Blood 130 (26): 2829-2837, 2017. [PUBMED Abstract]
- Evens AM, Advani RH, Helenowski IB, et al.: Multicenter Phase II Study of Sequential Brentuximab Vedotin and Doxorubicin, Vinblastine, and Dacarbazine Chemotherapy for Older Patients With Untreated Classical Hodgkin Lymphoma. J Clin Oncol 36 (30): 3015-3022, 2018. [PUBMED Abstract]
- Böll B, Goergen H, Behringer K, et al.: Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood 127 (18): 2189-92, 2016. [PUBMED Abstract]
- Herst J, Crump M, Baldassarre FG, et al.: Management of Early-stage Hodgkin Lymphoma: A Practice Guideline. Clin Oncol (R Coll Radiol) 29 (1): e5-e12, 2017. [PUBMED Abstract]
- Dühmke E, Franklin J, Pfreundschuh M, et al.: Low-dose radiation is sufficient for the noninvolved extended-field treatment in favorable early-stage Hodgkin's disease: long-term results of a randomized trial of radiotherapy alone. J Clin Oncol 19 (11): 2905-14, 2001. [PUBMED Abstract]
- Mendenhall NP, Rodrigue LL, Moore-Higgs GJ, et al.: The optimal dose of radiation in Hodgkin's disease: an analysis of clinical and treatment factors affecting in-field disease control. Int J Radiat Oncol Biol Phys 44 (3): 551-61, 1999. [PUBMED Abstract]
- Dabaja BS, Hoppe BS, Plastaras JP, et al.: Proton therapy for adults with mediastinal lymphomas: the International Lymphoma Radiation Oncology Group guidelines. Blood 132 (16): 1635-1646, 2018. [PUBMED Abstract]
Treatment of Early Favorable Classic HL
Patients are designated as having early favorable classic Hodgkin lymphoma (HL) when they have clinical stage I or stage II disease and none of the following adverse prognostic factors:
- B symptoms (unexplained fever ≥38°C, soaking night sweats, unexplained weight loss ≥10% within 6 months).
- Extranodal disease.
- Bulky disease (≥10 cm or >33% of the chest diameter on chest x-ray).
- Three or more sites of nodal involvement.
- Sedimentation rate of 50 mm/h or higher.
Treatment Options for Early Favorable Classic HL
Treatment options for early favorable classic HL include:
Chemotherapy with or without radiation therapy
Treatment options include:
- ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) for three to six cycles.[1]
- ABVD for two to four cycles plus involved-field radiation therapy (IFRT) (20 Gy or 30 Gy).
- Radiation therapy alone in certain circumstances (such as for older adults with absolute contraindications for using chemotherapy).[2,3]
Historically, radiation therapy alone was the primary treatment for patients with early favorable classic HL, often after confirmatory negative staging laparotomy.
The late mortality from solid tumors (especially in the lung, breast, gastrointestinal tract, and connective tissue) and cardiovascular disease makes radiation therapy a less-attractive option for the best-risk patients, who have the highest probability of cure and long-term survival.[4-8] Clinical trials have focused on regimens with chemotherapy and IFRT or with chemotherapy alone.[1]
Evidence (chemotherapy and/or radiation therapy):
For patients with early favorable classic HL, the following four trials established ABVD alone for four cycles or ABVD for two cycles plus 20 Gy of IFRT.
- A randomized, prospective trial from the National Cancer Institute of Canada involving 123 patients with early favorable classic HL compared ABVD for four to six cycles with subtotal nodal radiation.[9][Level of evidence A1]
- With a median follow-up of 11.3 years, no difference was observed in event-free survival rates (89% vs. 86%; P = .64) or in overall survival rates (OS) (98% vs. 98%; P = .95).
- A randomized study from the Milan Cancer Institute of patients with clinical early-stage HL compared 4 months of ABVD followed by IFRT with 4 months of ABVD followed by extended-field radiation therapy (EFRT).[10][Level of evidence B1]
- The results showed similar OS and freedom from progression of disease with a 10-year median follow-up, but the study had inadequate statistical power to determine noninferiority of IFRT versus EFRT.
- In the HD10 trial, the German Hodgkin Study Group (GHSG) randomly assigned 1,190 patients with early favorable HL to receive one of the following:[11,12][Level of evidence A1]
- Two cycles of ABVD plus 30 Gy of IFRT.
- Two cycles of ABVD plus 20 Gy of IFRT.
- Four cycles of ABVD plus 30 Gy of IFRT.
- Four cycles of ABVD plus 20 Gy of IFRT.
The following results were observed for the trial:
- With an 8.2-year median follow-up, no differences were observed (hazard ratio [HR], 1.0; 95% confidence interval [CI], 0.6–1.5) in 10-year progression-free survival (PFS) rates (87%) or OS rates (94%) for all four groups.
- A follow-up study by the GHSG (HD13 trial) compared modified versions of ABVD with elimination of dacarbazine, bleomycin, or both in combination with 30 Gy of radiation therapy in 1,502 patients with early favorable HL.[13]
- After 5 years, freedom from treatment failure was significantly worse when dacarbazine, bleomycin, or both were omitted.
- This trial suggests that ABVD remains the standard chemotherapy regimen.
Other trials have investigated the role of positron emission tomography (PET) scans for early favorable HL.
- Three prospective randomized trials (EORTC/LYSA/FIL H10 [NCT00433433][14,15]; RAPID [NCT00943423][16,17]; and GHSG HD16 [NCT00736320][18]) of 2,889 patients with early-stage disease investigated the use of PET‒computed tomography (CT) scans to modify therapy.[14-18]
- Among patients with early favorable HL who had negative PET-CT scan results (Deauville score of 1 or 2) after two or three cycles of ABVD, radiation therapy could be omitted with no significant loss of OS in all three trials.[14,16,18][Level of evidence B1]
However, two of the trials showed an increased risk of relapse when radiation therapy was omitted. In the GHSG HD16 trial, for the 628 patients with PET2-negative disease (PET after two cycles of ABVD), the 5-year PFS rate was 93.4% (95% CI, 90.4%–96.5%) with combined modality therapy and 86.1% (95% CI, 81.4%–90.0%) with ABVD alone (HR, 1.78; 95% CI, 1.02–3.12).[18] A subsequent analysis of the GHSG HD16 trial showed that most of the recurrences occurred in the proposed radiation field.[15] In the EORTC/LYSA/FIL H10 trial, the 10-year PFS rate was 98.8% with three cycles of ABVD plus radiation therapy and 85.4% with four cycles of ABVD without radiation therapy (HR, 13.2; 95% CI, 3.1–55.8; P < .001).[17]
In summary, this 7% to 13% difference in PFS without a difference in OS can be seen either as a mandate to combine radiation therapy with ABVD to avoid recurrences or as a rationale to give four or more cycles of ABVD when omitting radiation therapy.
- ABVD was given for three cycles (six doses) in the RAPID study,[16] for four cycles (eight doses) in the EORTC/LYSA/FIL H10 study,[14] and for two cycles (four doses) in the GHSG HD16 study [18] when applied without radiation therapy.
- None of the studies randomly assigned therapy for positive results from an interim PET-CT scan (Deauville score of 3, 4, or 5) after two or three cycles of ABVD because this occurred in only 15% to 25% of the patients studied. One of the studies (RAPID) added an extra cycle of ABVD and IFRT to 30 Gy,[16] another study (EORTC H10F) switched to BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone)–escalated therapy for two cycles plus involved nodal radiation therapy to 30 Gy,[14] and the other study (GHSG HD16) added IFRT to 30 Gy.[18]
In the RAPID study (NCT00943423), patients with postchemotherapy PET-CT Deauville scores of 5 (uptake ≥3 times maximum liver uptake) had inferior 5-year PFS rates (61.9%; 95% CI, 41.1%–82.7%) and 5-year OS rates (85.2%; 95% CI, 69.7%–100%) (P = .002) when compared with patients with Deauville scores of 1 to 4 (P < .001).[19]
- Among patients with early favorable HL who had negative PET-CT scan results (Deauville score of 1 or 2) after two or three cycles of ABVD, radiation therapy could be omitted with no significant loss of OS in all three trials.[14,16,18][Level of evidence B1]
Older patients with early favorable HL have also been studied.
- In 287 patients older than 60 years or with early favorable disease, a retrospective review of pulmonary toxicity in the HD10 and HD13 trials showed the following:[20]
- Two cycles of ABVD plus IFRT (137 patients): 2% pulmonary toxicity.
- Two cycles of AVD (omitting bleomycin) plus IFRT (82 patients): 2% pulmonary toxicity.
- Four cycles of ABVD plus IFRT (68 patients): 10% pulmonary toxicity.
For older patients (>60 years) with early favorable disease, when more than two cycles of ABVD are required, bleomycin may be omitted to avoid pulmonary toxicity.
Summary of early favorable classic HL:
- ABVD alone for three to four cycles is recommended for patients with early favorable classical HL when the interim PET-CT scan results are negative after two or three cycles of chemotherapy.[21] These patients are also unlikely to ever have a relapse, so routine CT scans are not recommended in follow-up.
- With positive interim PET-CT scan results, extra cycles of ABVD and involved nodal radiation therapy are recommended.
- A combined-modality approach with two cycles of ABVD and 20 Gy of IFRT can also be used for patients with early favorable classic HL.[21] In this situation, a PET-CT scan to assess response after completion of therapy would suffice.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Canellos GP, Abramson JS, Fisher DC, et al.: Treatment of favorable, limited-stage Hodgkin's lymphoma with chemotherapy without consolidation by radiation therapy. J Clin Oncol 28 (9): 1611-5, 2010. [PUBMED Abstract]
- Landgren O, Axdorph U, Fears TR, et al.: A population-based cohort study on early-stage Hodgkin lymphoma treated with radiotherapy alone: with special reference to older patients. Ann Oncol 17 (8): 1290-5, 2006. [PUBMED Abstract]
- Backstrand KH, Ng AK, Takvorian RW, et al.: Results of a prospective trial of mantle irradiation alone for selected patients with early-stage Hodgkin's disease. J Clin Oncol 19 (3): 736-41, 2001. [PUBMED Abstract]
- Dores GM, Metayer C, Curtis RE, et al.: Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol 20 (16): 3484-94, 2002. [PUBMED Abstract]
- Reinders JG, Heijmen BJ, Olofsen-van Acht MJ, et al.: Ischemic heart disease after mantlefield irradiation for Hodgkin's disease in long-term follow-up. Radiother Oncol 51 (1): 35-42, 1999. [PUBMED Abstract]
- Longo DL: Radiation therapy in Hodgkin disease: why risk a Pyrrhic victory? J Natl Cancer Inst 97 (19): 1394-5, 2005. [PUBMED Abstract]
- Swerdlow AJ, Higgins CD, Smith P, et al.: Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 99 (3): 206-14, 2007. [PUBMED Abstract]
- Engert A, Franklin J, Eich HT, et al.: Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. J Clin Oncol 25 (23): 3495-502, 2007. [PUBMED Abstract]
- Meyer RM, Gospodarowicz MK, Connors JM, et al.: ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med 366 (5): 399-408, 2012. [PUBMED Abstract]
- Bonadonna G, Bonfante V, Viviani S, et al.: ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin's disease: long-term results. J Clin Oncol 22 (14): 2835-41, 2004. [PUBMED Abstract]
- Engert A, Plütschow A, Eich HT, et al.: Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 363 (7): 640-52, 2010. [PUBMED Abstract]
- Sasse S, Bröckelmann PJ, Goergen H, et al.: Long-Term Follow-Up of Contemporary Treatment in Early-Stage Hodgkin Lymphoma: Updated Analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 Trials. J Clin Oncol 35 (18): 1999-2007, 2017. [PUBMED Abstract]
- Behringer K, Goergen H, Hitz F, et al.: Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet 385 (9976): 1418-27, 2015. [PUBMED Abstract]
- Raemaekers JM, André MP, Federico M, et al.: Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32 (12): 1188-94, 2014. [PUBMED Abstract]
- Baues C, Goergen H, Fuchs M, et al.: Involved-Field Radiation Therapy Prevents Recurrences in the Early Stages of Hodgkin Lymphoma in PET-Negative Patients After ABVD Chemotherapy: Relapse Analysis of GHSG Phase 3 HD16 Trial. Int J Radiat Oncol Biol Phys 111 (4): 900-906, 2021. [PUBMED Abstract]
- Radford J, Illidge T, Counsell N, et al.: Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med 372 (17): 1598-607, 2015. [PUBMED Abstract]
- Federico M, Fortpied C, Stepanishyna Y, et al.: Long-Term Follow-Up of the Response-Adapted Intergroup EORTC/LYSA/FIL H10 Trial for Localized Hodgkin Lymphoma. J Clin Oncol 42 (1): 19-25, 2024. [PUBMED Abstract]
- Fuchs M, Goergen H, Kobe C, et al.: Positron Emission Tomography-Guided Treatment in Early-Stage Favorable Hodgkin Lymphoma: Final Results of the International, Randomized Phase III HD16 Trial by the German Hodgkin Study Group. J Clin Oncol 37 (31): 2835-2845, 2019. [PUBMED Abstract]
- Barrington SF, Phillips EH, Counsell N, et al.: Positron Emission Tomography Score Has Greater Prognostic Significance Than Pretreatment Risk Stratification in Early-Stage Hodgkin Lymphoma in the UK RAPID Study. J Clin Oncol 37 (20): 1732-1741, 2019. [PUBMED Abstract]
- Böll B, Goergen H, Behringer K, et al.: Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood 127 (18): 2189-92, 2016. [PUBMED Abstract]
- Bröckelmann PJ, Sasse S, Engert A: Balancing risk and benefit in early-stage classical Hodgkin lymphoma. Blood 131 (15): 1666-1678, 2018. [PUBMED Abstract]
Treatment of Early Unfavorable Classic HL
Patients are designated as having early unfavorable classic Hodgkin lymphoma (HL) when they have clinical stage I or stage II disease and one or more of the following risk factors:
- B symptoms (unexplained fever ≥38°C, soaking night sweats, unexplained weight loss ≥10% within 6 months).
- Extranodal disease.
- Bulky disease (≥10 cm or >33% of the chest diameter on chest x-ray).
- Three or more sites of nodal involvement.
- Sedimentation rate of 50 mm/h or higher.
A retrospective review found that infradiaphragmatic early-stage disease appears to have an inferior outcome compared with the more frequent (>90%) supradiaphragmatic disease, with a decrement in overall survival (OS) rates of 6% (91.5% vs. 97.6%; P < .001).[1][Level of evidence C2]
Treatment Options for Early Unfavorable Classic HL
Treatment options for early unfavorable classic HL include:
Chemotherapy with or without radiation therapy
Treatment options include:[2,3]
See Table 4 for a description of the chemotherapy regimens used to treat HL.
Evidence (chemotherapy and radiation therapy):
- A randomized, prospective trial from the National Cancer Institute of Canada (NCIC) involving 276 patients with early unfavorable HL compared ABVD for four to six cycles with ABVD for two cycles plus extended-field radiation therapy (EFRT).[2][Level of evidence A1]
- With a median follow-up of 11.3 years, the freedom from progression score favored combined-modality therapy (86% vs. 94%; P = .006), but the OS rate was better for ABVD alone (92% vs. 81%; P = .04).
- The trend toward a worse survival for the combined-modality arm was attributed to excess secondary malignancies and cardiovascular deaths. In this trial, the EFRT used higher doses and significantly larger exposure to body sites than are employed in current practice.
- This trial established that six cycles of ABVD can be used alone and that long-term complications from radiation therapy can negate differences for progression-free survival (PFS).
- In the HD11 trial, the German Hodgkin Study Group (GHSG) randomly assigned 1,395 patients with early unfavorable HL to receive one of the following:
- Four cycles of ABVD plus 30 Gy of IFRT.
- Four cycles of ABVD plus 20 Gy of IFRT.
- Four cycles of BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) plus 30 Gy of IFRT.
- Four cycles of BEACOPP plus 20 Gy of IFRT.
The following results were observed:
- With an 8.8-year median follow-up, no differences were observed in OS rates (93%–96%) for all four groups.[8][Level of evidence A1]
- In the study arms using 30 Gy of IFRT, there was no difference in freedom from treatment failure between BEACOPP and ABVD (P = .65), but a significant difference against ABVD was seen for PFS when 20 Gy of IFRT was used (10-year PFS rate, 84% vs. 76%; hazard ratio (HR), 1.5; 95% confidence interval [CI], 1.0–2.1).[5][Level of evidence B1]
- In this trial, four cycles of ABVD plus 30 Gy of IFRT established this regimen as the preferred approach (or BEACOPP with 20 Gy of IFRT).
- In the HD14 trial, the GHSG randomly assigned 1,528 patients with early unfavorable HL to receive either four cycles of ABVD plus 30 Gy of IFRT or two cycles of escalated BEACOPP followed by two cycles of ABVD plus 30 Gy of IFRT.[6][Level of evidence A1]
- With a median follow-up of 43 months, no difference was observed in OS.
- In this trial, four cycles of ABVD plus 30 Gy of IFRT established this regimen as the preferred approach.
- In the H9-U trial, the European Organisation for Research and Treatment of Cancer–Groupe d'Étude des Lymphomes de l'Adulte (EORTC/GELA) randomly assigned 808 patients with early unfavorable disease (including 40% with bulky disease) to receive one of the following:[7][Level of evidence A1]:
- Six cycles of ABVD plus 36 Gy of IFRT.
- Four cycles of ABVD plus 36 Gy of IFRT.
- Four cycles of BEACOPP plus 36 Gy of IFRT.
The following results were observed:
- With a median follow-up of 64 months, no differences were observed (event-free survival rates, 89%–92%; P = .38; or OS rates, 91%–96%; P = .89).
- Based on toxicities, four cycles of ABVD plus IFRT was established as the preferred regimen.
- A multicenter nonrandomized study in 117 patients (most of whom had bulky disease) showed that four cycles of BV-AVD (brentuximab vedotin + doxorubicin, vinblastine, and dacarbazine) with or without involved-site radiation therapy is well-tolerated and effective.[9]
- With a median follow-up of 3.8 years, the overall 2-year PFS was 94% (95% CI, 89.7%–98.3%). The 2-year OS was 99.1% (97.3%– 100.0%).[9][Level of evidence C2]
- This pilot study requires confirmation, but the results may be reassuring when using the regimen for patients who cannot take bleomycin or need to limit anthracycline exposure.
Could the radiation therapy be omitted to minimize late morbidity and mortality from secondary solid tumors and from cardiovascular disease? [3]
- The NCIC study addressed this question in patients with early unfavorable HL. Although four to six cycles of ABVD alone had improved OS compared with a combined-modality approach, the use of EFRT in the combined-modality arm is excessive by current standards, and late effects will be magnified with these larger fields.[2] In addition, chemotherapy alone was 8% worse in freedom from disease progression compared with the combined-modality approach. An indirect comparison for using ABVD alone is that the 94% OS rate reported for patients with early unfavorable HL in the NCIC study [2] at 11 years is equivalent to the survival reported at 11 years in the GHSG's HD6 (NCT00002561), HD10 (NCT01399931), and HD11 (NCT0264953) trials using combined-modality therapy.[10] In addition, for the HD6 and HD10 trials, between the reports at 55 months and subsequently at 133 months, cardiovascular events doubled and solid tumor events tripled.[10]
- A retrospective analysis of 215 patients treated with ABVD and more contemporary radiation therapy (20 Gy–30 Gy, limited field) was compared with a cohort of 860 individuals matched for age, sex, geographical region, and major medical diseases.[11] Excess morbidity was still seen in terms of second malignancies, cardiovascular disease, and respiratory disease (HR, 1.5–7.6), but at a lower rate than in reports using regimens and doses from earlier decades.[11]
A Cochrane meta-analysis of 1,245 patients in five randomized clinical trials suggested improved survival for combined-modality therapy versus chemotherapy alone (HR, 0.40; 95% CI, 0.27–0.61).[12] However, the five randomized trials that were analyzed had inadequate follow-up to account for the late toxicities and increased mortality seen with radiation therapy after 10 years.
Other trials have investigated the role of positron emission tomography‒computed tomography (PET-CT) scans for patients with early unfavorable HL.
- A randomized prospective trial (EORTC HIOU) of 1,196 patients with early unfavorable HL investigated the use of PET-CT scans to modify therapy after two cycles of therapy.[13]
- Among the 815 patients with negative PET-CT findings (Deauville score of 1 or 2) after two cycles of ABVD, the patients randomly assigned to receive six cycles of ABVD had inferior PFS rates compared with patients who received four cycles of ABVD plus involved nodal radiation therapy (94.7% vs. 99.2%; P = .026), but no difference in OS.[Level of evidence B1]
- The use of ABVD for six cycles is acceptable in the absence of radiation therapy for patients with early unfavorable classic HL who have negative PET-CT results after two cycles of ABVD, if one can accept a 5% rate of increased relapse, with no decrement in OS after salvage therapy.
- In a follow-up report from this trial, 381 patients with positive PET-CT results (Deauville score of 3, 4, or 5) after two cycles of ABVD were randomly assigned to receive four cycles of ABVD plus 30 Gy of involved nodal radiation therapy versus two cycles of ABVD followed by two cycles of escalated BEACOPP plus 30 Gy of involved nodal radiation therapy.[14][Level of evidence A1]
- The 5-year PFS rate was 91% in the BEACOPP arm compared with 77% in the ABVD arm (P = .002).
- The 5-year OS rate was 96% in the BEACOPP arm compared with 89% in the ABVD arm (P = .02).
This trial supports adding escalated BEACOPP to ABVD for patients with early unfavorable classic HL who have positive PET-CT results after two cycles.
- A randomized prospective trial (GHSG HD17 [NCT01356680]) of 1,100 patients with early-stage unfavorable HL evaluated whether radiation therapy can be omitted in patients with a complete metabolic response (CMR) on PET-CT scan after two cycles of escalated BEACOPP and two cycles of regular-dose BEACOPP (2 + 2 regimen). Patients were randomly assigned to receive combined-modality therapy (n = 548) or PET4-guided therapy (n = 552). Combined-modality therapy included both the 2 + 2 regimen and involved-field radiation therapy. PET4-guided therapy included the 2 + 2 regimen for all patients and involved-node radiation therapy for the patients with a positive PET4 scan (n = 160). A total of 333 patients in the PET4-guided therapy group were PET4-negative and received chemotherapy alone.[15]
- With a median follow-up of 46.2 months, the 5-year PFS rate was 97.3% (95% CI, 94.5%–98.7%) for patients who received combined-modality therapy and 95.1% (95% CI, 92.0%–97.0%) for patients who received PET4-guided therapy (HR, 0.523; 95% CI, 0.23–1.21). The between-group difference was 2.2% (95% CI, -0.9% to 5.3%) and excluded the noninferiority margin of 8%.[15][Level of evidence B1]
- In the subgroup of PET4-negative patients who received chemotherapy alone, the difference in 5-year PFS was 1.7% (95% CI, -1.8% to 5.3%).
- Omitting radiation therapy for patients in CMR after four cycles of BEACOPP-based chemotherapy did not significantly impair PFS.
- A prospective phase II trial included 94 patients with early-stage (I/II) bulky disease (defined as mass >10 cm or >⅓ maximum intrathoracic diameter on chest x-ray). Patients received ABVD for two cycles, followed by interim PET (PET2) scan. PET-negative patients (78% of the total) were defined as Deauville 1, 2, or 3 and received two more cycles of ABVD. PET2-positive patients (Deauville 4 or 5, 22% of the total) received four cycles of escalated BEACOPP, followed by 30.6 Gy of IFRT.[16]
- With a median follow-up of 60 months, the 3-year PFS rate was 93.1% in PET2-negative patients and 89.7% in PET2-positive patients. The 3-year OS rate was 98.6% in PET2-negative patients and 94.4% in PET2-positive patients.[16][Level of evidence C3]
To summarize:
- Most of the trials support using four cycles of ABVD plus 30 Gy of IFRT or involved nodal radiation therapy.[17]
- ABVD alone for six cycles is a reasonable alternative despite a 5% to 6% decrement in PFS because the long-term toxicities of adding radiation therapy will affect OS, which is the most important patient outcome.[17]
- For patients with a positive PET-CT (usually Deauville 4 or 5) after two cycles of ABVD, adding brentuximab vedotin while eliminating bleomycin can be considered. BEACOPP or clinical trials investigating the addition of brentuximab vedotin or checkpoint inhibitors in this setting would be indicated.
- Radiation therapy may be omitted in patients with a negative PET-CT (Deauville 1) after two to four cycles of chemotherapy.
Patients with bulky disease (≥10 cm) or massive mediastinal involvement were excluded from most of the trials. On the basis of historical comparisons to chemotherapy or radiation therapy alone, these patients receive combined-modality therapy.[18-20][Level of evidence C2] A retrospective review published in a preliminary abstract reported on 194 patients with bulky disease who had PET-CT scans at the completion of chemotherapy; 112 of them had negative PET results (Deauville score of 1 or 2).[21] The observed 86% OS rate at 5 years suggests that radiation therapy can be excluded for patients with massive mediastinal disease who have negative PET-CT scan results after six cycles of therapy.[21][Level of evidence C2]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Sasse S, Goergen H, Plütschow A, et al.: Outcome of Patients With Early-Stage Infradiaphragmatic Hodgkin Lymphoma: A Comprehensive Analysis From the German Hodgkin Study Group. J Clin Oncol 36 (25): 2603-2611, 2018. [PUBMED Abstract]
- Meyer RM, Gospodarowicz MK, Connors JM, et al.: ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med 366 (5): 399-408, 2012. [PUBMED Abstract]
- Canellos GP, Abramson JS, Fisher DC, et al.: Treatment of favorable, limited-stage Hodgkin's lymphoma with chemotherapy without consolidation by radiation therapy. J Clin Oncol 28 (9): 1611-5, 2010. [PUBMED Abstract]
- Gunther JR, Fanale MA, Reddy JP, et al.: Treatment of Early-Stage Unfavorable Hodgkin Lymphoma: Efficacy and Toxicity of 4 Versus 6 Cycles of ABVD Chemotherapy With Radiation. Int J Radiat Oncol Biol Phys 96 (1): 110-8, 2016. [PUBMED Abstract]
- Eich HT, Diehl V, Görgen H, et al.: Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 28 (27): 4199-206, 2010. [PUBMED Abstract]
- von Tresckow B, Plütschow A, Fuchs M, et al.: Dose-intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German hodgkin study group HD14 trial. J Clin Oncol 30 (9): 907-13, 2012. [PUBMED Abstract]
- Fermé C, Thomas J, Brice P, et al.: ABVD or BEACOPPbaseline along with involved-field radiotherapy in early-stage Hodgkin Lymphoma with risk factors: Results of the European Organisation for Research and Treatment of Cancer (EORTC)-Groupe d'Étude des Lymphomes de l'Adulte (GELA) H9-U intergroup randomised trial. Eur J Cancer 81: 45-55, 2017. [PUBMED Abstract]
- Sasse S, Bröckelmann PJ, Goergen H, et al.: Long-Term Follow-Up of Contemporary Treatment in Early-Stage Hodgkin Lymphoma: Updated Analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 Trials. J Clin Oncol 35 (18): 1999-2007, 2017. [PUBMED Abstract]
- Kumar A, Casulo C, Advani RH, et al.: Brentuximab Vedotin Combined With Chemotherapy in Patients With Newly Diagnosed Early-Stage, Unfavorable-Risk Hodgkin Lymphoma. J Clin Oncol 39 (20): 2257-2265, 2021. [PUBMED Abstract]
- Meyer RM, Hoppe RT: Point/counterpoint: early-stage Hodgkin lymphoma and the role of radiation therapy. Blood 120 (23): 4488-95, 2012. [PUBMED Abstract]
- Lagerlöf I, Fohlin H, Enblad G, et al.: Limited, But Not Eliminated, Excess Long-Term Morbidity in Stage I-IIA Hodgkin Lymphoma Treated With Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine and Limited-Field Radiotherapy. J Clin Oncol 40 (13): 1487-1496, 2022. [PUBMED Abstract]
- Herbst C, Rehan FA, Skoetz N, et al.: Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin lymphoma. Cochrane Database Syst Rev (2): CD007110, 2011. [PUBMED Abstract]
- Raemaekers JM, André MP, Federico M, et al.: Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32 (12): 1188-94, 2014. [PUBMED Abstract]
- André MPE, Girinsky T, Federico M, et al.: Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol 35 (16): 1786-1794, 2017. [PUBMED Abstract]
- Borchmann P, Plütschow A, Kobe C, et al.: PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22 (2): 223-234, 2021. [PUBMED Abstract]
- LaCasce AS, Dockter T, Ruppert AS, et al.: Positron Emission Tomography-Adapted Therapy in Bulky Stage I/II Classic Hodgkin Lymphoma: CALGB 50801 (Alliance). J Clin Oncol 41 (5): 1023-1034, 2023. [PUBMED Abstract]
- Bröckelmann PJ, Sasse S, Engert A: Balancing risk and benefit in early-stage classical Hodgkin lymphoma. Blood 131 (15): 1666-1678, 2018. [PUBMED Abstract]
- Longo DL, Glatstein E, Duffey PL, et al.: Alternating MOPP and ABVD chemotherapy plus mantle-field radiation therapy in patients with massive mediastinal Hodgkin's disease. J Clin Oncol 15 (11): 3338-46, 1997. [PUBMED Abstract]
- Horning SJ, Hoppe RT, Breslin S, et al.: Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: mature results of a prospective clinical trial. J Clin Oncol 20 (3): 630-7, 2002. [PUBMED Abstract]
- Advani RH, Hong F, Fisher RI, et al.: Randomized Phase III Trial Comparing ABVD Plus Radiotherapy With the Stanford V Regimen in Patients With Stages I or II Locally Extensive, Bulky Mediastinal Hodgkin Lymphoma: A Subset Analysis of the North American Intergroup E2496 Trial. J Clin Oncol 33 (17): 1936-42, 2015. [PUBMED Abstract]
- Savage KJ: Advanced stage classical Hodgkin lymphoma patients with a negative PET-scan following treatment with ABVD have excellent outcomes without the need for consolidative radiotherapy regardless of disease bulk at presentation. [Abstract] Blood 126 (23): 579, 2015.
Treatment of Advanced Classic HL
The following adverse prognostic factors for advanced classic Hodgkin lymphoma (HL) have been combined into the International Prognostic Score (IPS) for advanced-stage HL:[1]
- Albumin level lower than 40 g/L.
- Hemoglobin level lower than 105 g/L.
- Male sex.
- Aged 45 years or older.
- Stage IV disease.
- White blood cell (WBC) count of 15 × 109/L or higher.
- Absolute lymphocyte count lower than 0.6 × 109/L or a lymphocyte count higher than 8% of the total WBC count.
| No. of Risk Factors | 5-Year FFP (%) | 5-Year OS (%) |
|---|---|---|
| FFP = freedom from progression; No. = number; OS = overall survival. | ||
| 0 | 88 | 98 |
| 1 | 84 | 97 |
| 2 | 80 | 92 |
| 3 | 74 | 91 |
| 4 | 67 | 88 |
| ≥5 | 62 | 73 |
Even the highest-risk patients in this index have a 5-year freedom from progression rate above 60% and a 5-year overall survival (OS) rate above 70%.[1]
Treatment Options for Advanced Classic HL
Treatment options for advanced classic HL include:
- Chemotherapy with or without immunotherapy or an antibody-drug conjugate.
- N-AVD (nivolumab [a checkpoint inhibitor] plus doxorubicin, vinblastine, and dacarbazine).
- BV-AVD (brentuximab vedotin [an antibody-drug conjugate directed against CD30] plus doxorubicin, vinblastine, and dacarbazine).
- ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).
Chemotherapy with or without nivolumab or brentuximab vedotin
The chemotherapy regimens N-AVD and BV-AVD are given for six cycles. These regimens have replaced ABVD, the previous standard regimen for three decades.[2,3] The ABVD regimen remains a viable option in cost-conscious settings.
See Table 4 for a description of the chemotherapy regimens used to treat HL.
Evidence (chemotherapy with or without nivolumab or brentuximab vedotin):
- A randomized prospective trial (NCT03907488) enrolled 994 patients with previously untreated advanced-stage HL. The study compared BV-AVD with N-AVD.[4]
- With a median follow-up of 2.1 years, the 2-year progression-free survival (PFS) rate favored N-AVD over BV-AVD at 92% (95% confidence interval [CI], 89%–94%) versus 83% (95% CI, 79%–86%) (hazard ratio [HR], 0.45; 95% CI, 0.30–0.65; P = .001).[4][Level of evidence B1]
- Treatment discontinuation due to side effects was twice as likely for patients who received BV-AVD (22% vs. 11%), mainly because of peripheral sensory neuropathy. Grade 2 or higher sensory peripheral neuropathy occurred in 32% of patients who received BV-AVD and 3% of patients who received N-AVD.
- A preplanned analysis, published in abstract form, included 97 patients aged 60 years or older. With a median follow-up of 12.1 months, the 1-year PFS favored N-AVD over BV-AVD at 93% versus 64% (HR, 0.35; 95% CI, 0.12–1.02; P = .022).[5] The BV-AVD regimen had substantially worse side effects including septicemia, peripheral sensory neuropathy, nausea, diarrhea, anorexia, and weight loss, compared with rash and hypothyroidism for N-AVD.[5]
- Based on the substantial PFS advantage across age, stage, and IPS score subgroups, as well as the reduced toxicity from avoiding bleomycin or brentuximab vedotin, N-AVD has become the treatment of choice for patients with stages III and IV HL.
- A randomized prospective trial (NCT01712490) included 1,334 patients with previously untreated advanced-stage HL. The study compared ABVD with BV-AVD.[6]
- With a median follow-up of 73 months, the 6-year OS rate was 93.9% (95% CI, 91.6%–95.5%) for patients who received BV-AVD and 89.4% (95% CI, 86.6%–91.7%) for patients who received ABVD (HR, 0.59; 95% CI, 0.40–0.88; P = .009).[6][Level of evidence A1]
- With a median follow-up of 73 months, the 6-year PFS rate was 82.3% (95% CI, 79.1%–85.0%) for patients who received BV-AVD and 74.5% (95% CI, 70.8%–77.7%) for patients who received ABVD (HR, 0.68; 95% CI, 0.53–0.86; P = .002).[6]
- Among patients who received BV-AVD, there was significantly more grade 3 or 4 peripheral neuropathy (67% vs. 43%); however, there was more than 80% partial or complete recovery, with a median time to resolution of 16 weeks for BV-AVD and 10 weeks for ABVD. Pulmonary toxicity led to 11 deaths in the ABVD arm.
- Although fertility was not directly assessed, pregnancies and live births subsequently occurred in both arms of the trial for female patients and female partners of the male patients.
- BV-AVD can cost 50 times more than ABVD (in 2018).[7]
- BV-AVD is a new standard of care for patients with advanced-stage classic HL.
- In multiple prospective trials and a meta-analysis, ABVD therapy for 6 to 8 months for patients with newly diagnosed advanced HL, showed equivalent OS when compared with other regimens (i.e., BEACOPP [bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone], escalated BEACOPP, Stanford V [doxorubicin, vinblastine, mechlorethamine, etoposide, vincristine, bleomycin, and prednisone], and MOPP-ABV [mechlorethamine, vincristine, procarbazine prednisone/doxorubicin, bleomycin, and vinblastine]).[8-15][Level of evidence A1]
Multiple studies have addressed the role of radiation therapy consolidation after induction chemotherapy for advanced-stage HL.
- Three prospective randomized trials did not show a benefit in OS from the addition of consolidative radiation therapy to chemotherapy for patients with advanced-stage disease.[16-18][Level of evidence A1]
- In a meta-analysis of 1,740 patients treated in 14 different trials, no improvement was observed in 10-year OS for patients with advanced-stage HL who received combined-modality therapy compared with chemotherapy alone.[19][Level of evidence C1]
- No survival advantage is known for the use of radiation consolidation for patients with massive mediastinal disease and advanced-stage disease.[20]
A randomized prospective trial with a median follow-up of 5.9 years included 320 patients with advanced-stage HL and a large nodal mass (≥5 cm). Patients were randomly assigned to receive radiation therapy or no further treatment after six cycles of ABVD. For patients with a complete metabolic response on positron emission tomography (PET)–computed tomography (CT) after six cycles of ABVD, there was no difference in the 6-year PFS rate for patients who received radiation therapy (91%; 95% CI, 84%–99%) versus patients who received no further treatment (95%; 95% CI, 89%–100%, P = .62).[21][Level of evidence B1]
- The German Hodgkin Lymphoma Study Group HD15 trial showed that a negative PET scan after induction therapy with BEACOPP (escalated or every 14 days) for advanced-stage HL was highly predictive for a good outcome, even with the omission of consolidative radiation therapy (negative predictive value for PET was 94% [95% CI, 91%–97%]).[22] In the German Hodgkin Study Group HD18 trial (NCT00515554), PET scan negativity after two cycles (PET2) of escalated BEACOPP allowed reduction to four cycles of therapy instead of six or eight cycles because of the equivalent 5-year PFS (90.8% vs. 92.2%; difference 1.4%; 95% CI, -2.7 to 5.4).[23][Level of evidence B1] The HD18 trial established a Deauville score of 4 or 5 as PET2 positive based on a 3-year OS.[24]
Other trials have investigated the role of PET scans in patients with advanced classic HL.
- A randomized prospective trial of 1,214 patients with advanced-stage HL (RATHL [NCT00678327]) investigated the use of PET-CT scans after two cycles of ABVD to modify therapy.[25,26] Patients with negative findings from a PET-CT scan (Deauville score of 1, 2, or 3) were randomly assigned to receive four more cycles of ABVD versus four cycles of AVD (doxorubicin, vinblastine, and dacarbazine).
- With a median follow-up of 7.3 years for the 937 patients with negative PET-CT results, there was no difference in the 7-year OS rate (93.2%; 95% CI, 90.2%–95.3% for ABVD vs. 93.5%; 95% CI, 90.5%–95.5% for AVD).[26][Level of evidence A1]
- The absolute difference in the 3-year PFS rate (ABVD minus AVD) was 1.3% (95% CI, -3.0% to 4.7%), which falls within the predefined noninferiority margin. This meant that there was no PFS advantage for continuing bleomycin for PET-negative patients on the interim scan.
- However, pulmonary toxicity was worse in the continued ABVD arm, with significantly more grade 3 or 4 respiratory events and worsened long-term diffusing capacity of the lung for carbon monoxide levels persisting beyond 1 year.
- This study concluded that bleomycin may be omitted after the second cycle of ABVD if findings from the PET-CT scan are negative (Deauville score of 1, 2, or 3).
- The patients with positive PET-CT scan results (Deauville score of 4 or 5) after two cycles of ABVD received BEACOPP.
- With a median follow-up of 41 months for the 172 patients with positive PET-CT results, the 3-year PFS rate was 67.5% and the OS rate was 87.8%
- This trial did not establish that switching to BEACOPP was superior to remaining on ABVD.
- In a nonrandomized trial (SWOG S0816 [NCT00822120]), 336 patients with advanced HL received two cycles of ABVD and then were evaluated by PET scan.[27] PET2–negative patients (Deauville score of 1 to 3) completed four more cycles of ABVD, while the 60 PET2–positive patients (18% of total) were switched to escalated BEACOPP.
- With a median follow-up of 5.9 years, the 5-year PFS rate for the PET2–positive patients was 66% (95% CI, 52%–76%).[27][Level of evidence C3]
Older patients with advanced-stage HL have also been studied.
- In a multicenter phase II study, 48 patients older than 60 years, of whom 81% had advanced-stage disease, received brentuximab vedotin for two consecutive doses, followed by six cycles of AVD, followed by four more doses of brentuximab vedotin.[28]
- The 2-year event-free survival rate was 80%, PFS rate was 84%, and OS rate was 93%.[28][Level of evidence C3]
- Grade 3 or 4 toxicity was experienced by 42% of patients.
Summary of advanced-stage classic HL:
- For patients with advanced-stage HL, six cycles of N-AVD is now the standard approach. In situations where immunotherapy might be contraindicated (such as active vasculitis or inflammatory colitis), BV-AVD is a good option.
- When the financial toxicity of N-AVD or BV-AVD precludes their use (such as in a nation with constrained health care options), ABVD is still a reasonable and cost-effective approach. For patients with negative PET-CT scan results after the second cycle of ABVD, bleomycin may be omitted from the chemotherapy regimen with little loss of efficacy and improvement in tolerability.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Moccia AA, Donaldson J, Chhanabhai M, et al.: International Prognostic Score in advanced-stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol 30 (27): 3383-8, 2012. [PUBMED Abstract]
- Connors JM, Jurczak W, Straus DJ, et al.: Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma. N Engl J Med 378 (4): 331-344, 2018. [PUBMED Abstract]
- Straus DJ, Długosz-Danecka M, Alekseev S, et al.: Brentuximab vedotin with chemotherapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study. Blood 135 (10): 735-742, 2020. [PUBMED Abstract]
- Herrera AF, LeBlanc M, Castellino SM, et al.: Nivolumab+AVD in Advanced-Stage Classic Hodgkin's Lymphoma. N Engl J Med 391 (15): 1379-1389, 2024. [PUBMED Abstract]
- Rutherford SC, Li H, Herrera AF, et al.: Nivolumab-AVD is better tolerated and improves progression-free survival compared to Bv-AVD in older patients (aged ≥60 years) with advanced stage Hodgkin lymphoma enrolled on SWOG S1826. [Abstract] Blood 142 (Suppl 1): A-624, 181, 2023.
- Ansell SM, Radford J, Connors JM, et al.: Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin's Lymphoma. N Engl J Med 387 (4): 310-320, 2022. [PUBMED Abstract]
- Huntington SF, von Keudell G, Davidoff AJ, et al.: Cost-Effectiveness Analysis of Brentuximab Vedotin With Chemotherapy in Newly Diagnosed Stage III and IV Hodgkin Lymphoma. J Clin Oncol : JCO1800122, 2018. [PUBMED Abstract]
- Canellos GP, Niedzwiecki D: Long-term follow-up of Hodgkin's disease trial. N Engl J Med 346 (18): 1417-8, 2002. [PUBMED Abstract]
- Duggan DB, Petroni GR, Johnson JL, et al.: Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol 21 (4): 607-14, 2003. [PUBMED Abstract]
- Federico M, Luminari S, Iannitto E, et al.: ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 27 (5): 805-11, 2009. [PUBMED Abstract]
- Viviani S, Zinzani PL, Rambaldi A, et al.: ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med 365 (3): 203-12, 2011. [PUBMED Abstract]
- Bauer K, Skoetz N, Monsef I, et al.: Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev (8): CD007941, 2011. [PUBMED Abstract]
- Chisesi T, Bellei M, Luminari S, et al.: Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin's lymphoma: a study from the Intergruppo Italiano Linfomi. J Clin Oncol 29 (32): 4227-33, 2011. [PUBMED Abstract]
- Carde P, Karrasch M, Fortpied C, et al.: Eight Cycles of ABVD Versus Four Cycles of BEACOPPescalated Plus Four Cycles of BEACOPPbaseline in Stage III to IV, International Prognostic Score ≥ 3, High-Risk Hodgkin Lymphoma: First Results of the Phase III EORTC 20012 Intergroup Trial. J Clin Oncol 34 (17): 2028-36, 2016. [PUBMED Abstract]
- Mounier N, Brice P, Bologna S, et al.: ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol 25 (8): 1622-8, 2014. [PUBMED Abstract]
- Fabian CJ, Mansfield CM, Dahlberg S, et al.: Low-dose involved field radiation after chemotherapy in advanced Hodgkin disease. A Southwest Oncology Group randomized study. Ann Intern Med 120 (11): 903-12, 1994. [PUBMED Abstract]
- Aleman BM, Raemaekers JM, Tirelli U, et al.: Involved-field radiotherapy for advanced Hodgkin's lymphoma. N Engl J Med 348 (24): 2396-406, 2003. [PUBMED Abstract]
- Fermé C, Mounier N, Casasnovas O, et al.: Long-term results and competing risk analysis of the H89 trial in patients with advanced-stage Hodgkin lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte (GELA). Blood 107 (12): 4636-42, 2006. [PUBMED Abstract]
- Loeffler M, Brosteanu O, Hasenclever D, et al.: Meta-analysis of chemotherapy versus combined modality treatment trials in Hodgkin's disease. International Database on Hodgkin's Disease Overview Study Group. J Clin Oncol 16 (3): 818-29, 1998. [PUBMED Abstract]
- Brice P, Colin P, Berger F, et al.: Advanced Hodgkin disease with large mediastinal involvement can be treated with eight cycles of chemotherapy alone after a major response to six cycles of chemotherapy: a study of 82 patients from the Groupes d'Etudes des Lymphomes de l'Adulte H89 trial. Cancer 92 (3): 453-9, 2001. [PUBMED Abstract]
- Gallamini A, Rossi A, Patti C, et al.: Consolidation Radiotherapy Could Be Safely Omitted in Advanced Hodgkin Lymphoma With Large Nodal Mass in Complete Metabolic Response After ABVD: Final Analysis of the Randomized GITIL/FIL HD0607 Trial. J Clin Oncol 38 (33): 3905-3913, 2020. [PUBMED Abstract]
- Kobe C, Dietlein M, Franklin J, et al.: Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood 112 (10): 3989-94, 2008. [PUBMED Abstract]
- Borchmann P, Goergen H, Kobe C, et al.: PET-guided treatment in patients with advanced-stage Hodgkin's lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 390 (10114): 2790-2802, 2018. [PUBMED Abstract]
- Kobe C, Goergen H, Baues C, et al.: Outcome-based interpretation of early interim PET in advanced-stage Hodgkin lymphoma. Blood 132 (21): 2273-2279, 2018. [PUBMED Abstract]
- Johnson P, Federico M, Kirkwood A, et al.: Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin's Lymphoma. N Engl J Med 374 (25): 2419-29, 2016. [PUBMED Abstract]
- Luminari S, Fossa A, Trotman J, et al.: Long-Term Follow-Up of the Response-Adjusted Therapy for Advanced Hodgkin Lymphoma Trial. J Clin Oncol 42 (1): 13-18, 2024. [PUBMED Abstract]
- Stephens DM, Li H, Schöder H, et al.: Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood 134 (15): 1238-1246, 2019. [PUBMED Abstract]
- Evens AM, Advani RH, Helenowski IB, et al.: Multicenter Phase II Study of Sequential Brentuximab Vedotin and Doxorubicin, Vinblastine, and Dacarbazine Chemotherapy for Older Patients With Untreated Classical Hodgkin Lymphoma. J Clin Oncol 36 (30): 3015-3022, 2018. [PUBMED Abstract]
Treatment of Recurrent Classic HL
More than one-half of all patients with recurrent Hodgkin lymphoma (HL) can achieve long-term disease-free survival (DFS), or even cure, using reinduction therapy followed by stem cell/bone marrow transplant consolidation.[1] In this regard, the disease follows a 75% rule: 75% of patients attain a clinical complete remission with salvage therapy reinduction, and then 75% of patients who undergo autologous stem cell transplant (SCT) are free of disease at 4 years. Poor prognostic factors include:[2-4]
- Primary refractory disease (worst prognosis).
- Relapse less than 12 months after initial therapy. Among patients who initially present with early-stage favorable disease that relapses, more than 75% have a relapse more than 12 months after diagnosis.[5]
- Inability to attain a clinical complete remission after reinduction (i.e., positron emission tomography‒computed tomography [PET-CT] scan results are positive with a Deauville score of 4 or 5 followed by subsequent progression in the size and/or sites of disease).
- B symptoms at relapse.
- Extranodal disease at relapse.
- More than two previous salvage regimens received.
Treatment Options for Recurrent Classic HL
Treatment options for recurrent classic HL include:
Pembrolizumab or nivolumab (alone or with chemotherapy)
The anti-programmed cell death-1 (PD-1) monoclonal antibodies pembrolizumab and nivolumab are immune checkpoint inhibitors.
Evidence: (pembrolizumab):
- In a phase II trial of 37 patients with relapsed or refractory disease, patients received three cycles of pembrolizumab with two cycles of ICE chemotherapy (ifosfamide, carboplatin, and etoposide) every 21 days prior to autologous SCT.[6][Level of evidence C3]
- The complete response rate was 86.5% (95% confidence interval [CI], 71.2%–95.5%), and the overall response rate was 97.3%. There was no impairment in stem cell mobilization.
- A phase II trial included 39 patients with transplant-eligible relapsed or refractory disease. Patients received pembrolizumab with GVD chemotherapy (gemcitabine, vinorelbine, and liposomal doxorubicin).[7]
- With a median follow-up of 13.5 months, the overall response rate was 100%, and the complete response rate was 95%.[7][Level of evidence C3]
- Thirty-six patients (35%) proceeded to autologous SCT consolidation.
- A prospective randomized trial included 304 patients with relapsed or refractory disease who were ineligible for or had a relapse after autologous SCT. Patients were assigned to receive either pembrolizumab or brentuximab vedotin.[8]
- With a median follow-up of 25.7 months, the median progression-free survival (PFS) for patients who received pembrolizumab was 13.2 months (95% CI, 10.9–19.4) versus 8.3 months (95% CI, 5.7–8.8) for patients who received brentuximab vedotin (hazard ratio [HR], 0.65; 95% CI, 0.48–0.88; P = .0027).[8][Level of evidence B1]
- Serious treatment-related adverse events occurred in 16% of patients who received pembrolizumab and 11% of patients who received brentuximab vedotin.
- Studies of patients with relapsed HL treated with pembrolizumab reported the following:[9,10][Level of evidence C3]
- The overall response rate was 64% to 74%, with a complete response rate of 22.4% (95% CI, 6.9%–28.6%).
- Pembrolizumab was well tolerated by patients and can be used to achieve a clinical complete remission before autologous or allogeneic SCT.
- The U.S. Food and Drug Administration (FDA) approved pembrolizumab for use in cases of refractory disease or relapse after three or more lines of therapy.
Evidence (nivolumab alone or nivolumab plus ICE):
- Studies of patients with relapsed HL treated with nivolumab reported the following:[11-13][Level of evidence C3]
- The overall response rate was 65% to 87% and the complete response rate was 16% to 28%, with response durations usually exceeding 1 year for patients with heavily pretreated, relapsed disease.
- Nivolumab was well tolerated by patients and can be used to achieve a clinical complete remission before autologous or allogeneic SCT.
- The FDA approved nivolumab for use after both relapse from SCT and previous exposure to brentuximab vedotin. Nivolumab is also approved if the patient has received three different previous treatments, including SCT.
- In a phase II trial, nivolumab was given for 3 months. Patients who achieved a complete response proceeded to autologous SCT, while patients with disease in partial response or less received NICE (nivolumab, ifosfamide, carboplatin, and etoposide).[14]
- Nivolumab induction was given to 34 patients, and 9 patients needed NICE because the complete response rate was 71% for nivolumab. After all therapy, the overall response rate was 93%, and the complete response rate was 91%. The 2-year PFS rate was 72%, and the 2-year OS rate was 95%.[14][Level of evidence C3]
Brentuximab vedotin
Brentuximab vedotin is an antibody-drug conjugate directed against CD30.[15-17] CD30 is a target for therapy because it is expressed on malignant Reed-Sternberg cells of HL but has limited expression on normal cells. Brentuximab vedotin is well tolerated by patients and can be used to achieve a clinical complete response before autologous or allogeneic SCT.
Evidence (brentuximab vedotin):
- In multiple trials for patients with relapsed disease, including one trial performed after allogeneic SCT, the following results were observed:
- For patients with relapsing disease, response rates were approximately 75%. Complete remission rates were approximately 50% and median PFS was 4 to 8 months.[15-19][Level of evidence C3]
- Twenty-seven previously untreated patients older than 60 years, judged by the investigator to be in poor condition and unable to undergo chemotherapy, received brentuximab vedotin.[20]
- A 92% overall response rate and 73% complete remission rate were reported.[20][Level of evidence C3]
- Retreatment with brentuximab vedotin was successful in patients with relapsed disease, with a response rate of 60%.[21][Level of evidence C3]
- For 329 patients at high risk of residual HL after SCT, the double-blind AETHERA trial (NCT01100502) evaluated brentuximab vedotin versus placebo.[22,23]
- With a median follow-up of 5.0 years, the 5-year PFS rate for brentuximab vedotin was 59% (95% CI, 51%–66%) versus 41% (95% CI, 33%–49%) for placebo (HR, 0.521; 95% CI, 0.379–0.717).[22,23][Level of evidence B1]
- The 16-month treatment duration after transplant was not achieved by most patients because they developed progressive peripheral neuropathy, which was partially reversible after discontinuation of brentuximab vedotin.
- In two phase I/II studies, 120 patients with relapsed or refractory HL received brentuximab vedotin and bendamustine.[24]
- After two cycles, the objective response rates were 93% and 78%, and the complete remission rates were 74% and 32%.[24,25][Level of evidence C3]
Brentuximab vedotin plus nivolumab
Evidence (brentuximab vedotin plus nivolumab):
- In a phase II trial, 91 patients with relapsed or refractory HL received brentuximab vedotin and nivolumab.[26] Prior brentuximab vedotin therapy was allowed if the patient was not resistant or intolerant to the drug.
- With a median follow-up of 34.3 months, the overall response rate was 85%, and the complete response rate was 67%. The 3-year PFS rate was 77% (95% CI, 65%–86%) for all patients and 91% (95% CI, 79%–96%) for those who received autologous SCT. The 3-year OS rate was 93% (95% CI, 85%–97%).[26][Level of evidence C3]
- In this trial, 16% of patients had adverse events that required treatment with steroids.
- In a phase I/II study of 59 patients with relapsed or refractory HL, the combination of nivolumab and brentuximab vedotin was well tolerated (<10% of patients required systemic steroids).[27][Level of evidence C3]
- With a median follow-up of 28.9 months, the 18-month PFS rate was 94% (95% CI, 84%–98%).
- Adverse events included peripheral neuropathy (53%), neutropenia (42%), and immune-related events requiring corticosteroids (29%).[27]
Chemotherapy with stem cell transplant
Patients whose HL relapses after initial combination chemotherapy can undergo reinduction with the same or another chemotherapy regimen followed by high-dose chemotherapy and autologous bone marrow or peripheral stem cell or allogeneic bone marrow rescue.[1,28-31] This therapy has resulted in 3- to 4-year DFS rates of up to 50%. Patients who are responsive to reinduction therapy may have a better prognosis after subsequent autologous SCT; in one analysis, the 3-year event-free survival (EFS) rate was 80% with negative PET-CT scan results and 29% with positive PET-CT scan results.[32]
Patients who do not respond to induction chemotherapy (about 20%‒25% of all presenting patients) have survival rates lower than 10% at 8 years.[3] For these patients, high-dose chemotherapy and autologous bone marrow or peripheral stem cell or allogeneic bone marrow rescue [28,29,33-35] have resulted in 5-year DFS rates of around 25% to 30%, but selection bias clearly influences these numbers.[28,29,34,36,37]
In a retrospective review of 105 patients, those older than 60 years fared better with a combination of chemotherapy and salvage radiation therapy than with the use of intensified transplant consolidation.[38][Level of evidence C3]
The use of HLA-matched sibling marrow (allogeneic transplant) results in lower relapse rates, but the benefit may be offset by increased toxic effects.[28,39,40] Reduced-intensity conditioning for allogeneic SCT is also under clinical evaluation.[41-43]
Evidence (chemotherapy with SCT):
- A randomized trial compared aggressive conventional chemotherapy versus high-dose chemotherapy with autologous hematopoietic SCT for relapsed chemosensitive HL.[44][Level of evidence B1]
- This trial showed improvement in freedom from treatment failure at 3 years for the transplant arm (55%) versus the chemotherapy-alone arm (34%).[44]
- No difference was observed in overall survival (OS).
- A Cochrane meta-analysis concluded that autologous SCT after reinduction chemotherapy improves relapse-free survival by 20% to 30% over chemotherapy alone, but without an OS benefit.[45][Level of evidence B1]
- In three retrospective reviews of patients who
underwent autologous bone marrow transplant (BMT) for relapsed or refractory disease, a comparison was made between
those who received involved-field radiation therapy (IFRT) for residual masses after
high-dose therapy and those who received no further treatment.[46-48]
- Those who received IFRT had decreased local disease recurrence.
- Normalization of fluorine F 18-fludeoxyglucose PET-CT scans after reinduction therapy predicted a much better outcome after SCT, with an EFS rate of 80% versus 29% in one phase II trial.[32][Level of evidence C2]
After completion of autologous SCT for recurrent HL, 329 patients were randomly assigned to receive brentuximab vedotin or placebo in a double-blind trial (AETHERA [NCT01100502]).[22,23]
- With a median follow-up of 5.0 years, the 5-year PFS rate for brentuximab vedotin was 59% (95% CI, 51%–66%) versus 41% (95% CI, 33%–49%) for placebo (HR, 0.521; 95% CI, 0.379–0.717).[22,23][Level of evidence B1]
- The 16-month treatment duration after transplant was not achieved by most patients because they developed progressive peripheral neuropathy, which was partially reversible after discontinuation of brentuximab vedotin.
- It is unclear whether the results of this trial are applicable when brentuximab vedotin is employed before transplant, such as during reinduction after relapse or during initial therapy (presently under clinical evaluation).
A phase II trial reported a response rate higher than 50% for patients with relapsing disease after autologous BMT.[49][Level of evidence C3] For patients with recurrent disease after autologous BMT, weekly vinblastine therapy has provided palliation with minimal toxic effects.[50][Level of evidence C3]
Combination chemotherapy
For patients who experience a relapse after initial combination chemotherapy, prognosis is determined more by the duration of the first remission than by the specific induction or salvage combination chemotherapy regimen. Patients whose initial remission after chemotherapy was longer than 1 year (late relapse) have long-term survival rates of 22% to 71% with salvage chemotherapy.[2-4,51-53] Patients whose initial remission after chemotherapy was shorter than 1 year (early relapse) do much worse and have long-term survival rates of 11% to 46%.[2,3,54]
It is rare to see a patient who received only radiation therapy for initial treatment, but patients who experience a relapse after initial wide-field, high-dose radiation therapy have a good prognosis. Combination chemotherapy results in 10-year DFS rates of 57% to 81% and OS rates of 57% to 89%.[2,55-57]
Radiation therapy
For the small subgroup of patients with only limited nodal recurrence following initial chemotherapy, radiation therapy with or without additional chemotherapy may provide long-term survival for about 50% of these highly selected patients.[58,59]
Summary for sequencing therapies for recurrent classic HL
- Patients whose disease recurs who have not received brentuximab vedotin or a checkpoint inhibitor should consider the combination of nivolumab and brentuximab vedotin.[26,60]
- The combination of pembrolizumab plus ICE chemotherapy,[6] nivolumab plus ICE chemotherapy,[14] or pembrolizumab plus GVD chemotherapy [7] is an effective induction therapy prior to autologous SCT.
- Consider allogeneic SCT for patients with primary refractory disease who achieved partial response or complete remission on salvage therapy.
- Checkpoint inhibitors alone are useful palliative agents for older patients or patients with comorbidities that preclude SCT.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Holmberg L, Maloney DG: The role of autologous and allogeneic hematopoietic stem cell transplantation for Hodgkin lymphoma. J Natl Compr Canc Netw 9 (9): 1060-71, 2011. [PUBMED Abstract]
- Josting A, Franklin J, May M, et al.: New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol 20 (1): 221-30, 2002. [PUBMED Abstract]
- Bonfante V, Santoro A, Viviani S, et al.: Outcome of patients with Hodgkin's disease failing after primary MOPP-ABVD. J Clin Oncol 15 (2): 528-34, 1997. [PUBMED Abstract]
- Garcia-Carbonero R, Paz-Ares L, Arcediano A, et al.: Favorable prognosis after late relapse of Hodgkin's disease. Cancer 83 (3): 560-5, 1998. [PUBMED Abstract]
- Bröckelmann PJ, Müller H, Guhl T, et al.: Relapse After Early-Stage, Favorable Hodgkin Lymphoma: Disease Characteristics and Outcomes With Conventional or High-Dose Chemotherapy. J Clin Oncol 39 (2): 107-115, 2021. [PUBMED Abstract]
- Bryan LJ, Casulo C, Allen PB, et al.: Pembrolizumab Added to Ifosfamide, Carboplatin, and Etoposide Chemotherapy for Relapsed or Refractory Classic Hodgkin Lymphoma: A Multi-institutional Phase 2 Investigator-Initiated Nonrandomized Clinical Trial. JAMA Oncol 9 (5): 683-691, 2023. [PUBMED Abstract]
- Moskowitz AJ, Shah G, Schöder H, et al.: Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J Clin Oncol 39 (28): 3109-3117, 2021. [PUBMED Abstract]
- Kuruvilla J, Ramchandren R, Santoro A, et al.: Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 22 (4): 512-524, 2021. [PUBMED Abstract]
- Armand P, Shipp MA, Ribrag V, et al.: Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol 34 (31): 3733-3739, 2016. [PUBMED Abstract]
- Chen R, Zinzani PL, Lee HJ, et al.: Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 134 (14): 1144-1153, 2019. [PUBMED Abstract]
- Ansell SM, Lesokhin AM, Borrello I, et al.: PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372 (4): 311-9, 2015. [PUBMED Abstract]
- Younes A, Santoro A, Shipp M, et al.: Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17 (9): 1283-94, 2016. [PUBMED Abstract]
- Armand P, Engert A, Younes A, et al.: Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol 36 (14): 1428-1439, 2018. [PUBMED Abstract]
- Mei MG, Lee HJ, Palmer JM, et al.: Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood 139 (25): 3605-3616, 2022. [PUBMED Abstract]
- Gopal AK, Ramchandren R, O'Connor OA, et al.: Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood 120 (3): 560-8, 2012. [PUBMED Abstract]
- Younes A, Bartlett NL, Leonard JP, et al.: Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363 (19): 1812-21, 2010. [PUBMED Abstract]
- Younes A, Gopal AK, Smith SE, et al.: Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 30 (18): 2183-9, 2012. [PUBMED Abstract]
- Chen R, Palmer JM, Thomas SH, et al.: Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood 119 (26): 6379-81, 2012. [PUBMED Abstract]
- Bazarbachi A, Boumendil A, Finel H, et al.: Brentuximab vedotin for recurrent Hodgkin lymphoma after allogeneic hematopoietic stem cell transplantation: A report from the EBMT Lymphoma Working Party. Cancer 125 (1): 90-98, 2019. [PUBMED Abstract]
- Forero-Torres A, Holkova B, Goldschmidt J, et al.: Phase 2 study of frontline brentuximab vedotin monotherapy in Hodgkin lymphoma patients aged 60 years and older. Blood 126 (26): 2798-804, 2015. [PUBMED Abstract]
- Bartlett NL, Chen R, Fanale MA, et al.: Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol 7: 24, 2014. [PUBMED Abstract]
- Moskowitz CH, Nademanee A, Masszi T, et al.: Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385 (9980): 1853-62, 2015. [PUBMED Abstract]
- Moskowitz CH, Walewski J, Nademanee A, et al.: Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood 132 (25): 2639-2642, 2018. [PUBMED Abstract]
- LaCasce AS, Bociek RG, Sawas A, et al.: Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood 132 (1): 40-48, 2018. [PUBMED Abstract]
- O'Connor OA, Lue JK, Sawas A, et al.: Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial. Lancet Oncol 19 (2): 257-266, 2018. [PUBMED Abstract]
- Advani RH, Moskowitz AJ, Bartlett NL, et al.: Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood 138 (6): 427-438, 2021. [PUBMED Abstract]
- Herrera AF, Chen L, Nieto Y, et al.: Brentuximab vedotin plus nivolumab after autologous haematopoietic stem-cell transplantation for adult patients with high-risk classic Hodgkin lymphoma: a multicentre, phase 2 trial. Lancet Haematol 10 (1): e14-e23, 2023. [PUBMED Abstract]
- Akpek G, Ambinder RF, Piantadosi S, et al.: Long-term results of blood and marrow transplantation for Hodgkin's lymphoma. J Clin Oncol 19 (23): 4314-21, 2001. [PUBMED Abstract]
- Tarella C, Cuttica A, Vitolo U, et al.: High-dose sequential chemotherapy and peripheral blood progenitor cell autografting in patients with refractory and/or recurrent Hodgkin lymphoma: a multicenter study of the intergruppo Italiano Linfomi showing prolonged disease free survival in patients treated at first recurrence. Cancer 97 (11): 2748-59, 2003. [PUBMED Abstract]
- Shah GL, Moskowitz CH: Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood 131 (15): 1689-1697, 2018. [PUBMED Abstract]
- Martínez C, Gayoso J, Canals C, et al.: Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation as Alternative to Matched Sibling or Unrelated Donor Transplantation for Hodgkin Lymphoma: A Registry Study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol 35 (30): 3425-3432, 2017. [PUBMED Abstract]
- Moskowitz CH, Matasar MJ, Zelenetz AD, et al.: Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood 119 (7): 1665-70, 2012. [PUBMED Abstract]
- Fermé C, Mounier N, Diviné M, et al.: Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin's disease in relapse or failure after initial chemotherapy: results of the Groupe d'Etudes des Lymphomes de l'Adulte H89 Trial. J Clin Oncol 20 (2): 467-75, 2002. [PUBMED Abstract]
- Gopal AK, Metcalfe TL, Gooley TA, et al.: High-dose therapy and autologous stem cell transplantation for chemoresistant Hodgkin lymphoma: the Seattle experience. Cancer 113 (6): 1344-50, 2008. [PUBMED Abstract]
- Morschhauser F, Brice P, Fermé C, et al.: Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin's lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol 26 (36): 5980-7, 2008. [PUBMED Abstract]
- Nademanee A, O'Donnell MR, Snyder DS, et al.: High-dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin's disease: results in 85 patients with analysis of prognostic factors. Blood 85 (5): 1381-90, 1995. [PUBMED Abstract]
- Horning SJ, Chao NJ, Negrin RS, et al.: High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin's disease: analysis of the Stanford University results and prognostic indices. Blood 89 (3): 801-13, 1997. [PUBMED Abstract]
- Böll B, Goergen H, Arndt N, et al.: Relapsed hodgkin lymphoma in older patients: a comprehensive analysis from the German hodgkin study group. J Clin Oncol 31 (35): 4431-7, 2013. [PUBMED Abstract]
- Milpied N, Fielding AK, Pearce RM, et al.: Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin's disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol 14 (4): 1291-6, 1996. [PUBMED Abstract]
- Gajewski JL, Phillips GL, Sobocinski KA, et al.: Bone marrow transplants from HLA-identical siblings in advanced Hodgkin's disease. J Clin Oncol 14 (2): 572-8, 1996. [PUBMED Abstract]
- Kuruvilla J, Pintilie M, Stewart D, et al.: Outcomes of reduced-intensity conditioning allo-SCT for Hodgkin's lymphoma: a national review by the Canadian Blood and Marrow Transplant Group. Bone Marrow Transplant 45 (7): 1253-5, 2010. [PUBMED Abstract]
- Peggs KS, Kayani I, Edwards N, et al.: Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin's lymphoma. J Clin Oncol 29 (8): 971-8, 2011. [PUBMED Abstract]
- Genadieva-Stavrik S, Boumendil A, Dreger P, et al.: Myeloablative versus reduced intensity allogeneic stem cell transplantation for relapsed/refractory Hodgkin's lymphoma in recent years: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Ann Oncol 27 (12): 2251-2257, 2016. [PUBMED Abstract]
- Schmitz N, Pfistner B, Sextro M, et al.: Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet 359 (9323): 2065-71, 2002. [PUBMED Abstract]
- Rancea M, Monsef I, von Tresckow B, et al.: High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev 6: CD009411, 2013. [PUBMED Abstract]
- Mundt AJ, Sibley G, Williams S, et al.: Patterns of failure following high-dose chemotherapy and autologous bone marrow transplantation with involved field radiotherapy for relapsed/refractory Hodgkin's disease. Int J Radiat Oncol Biol Phys 33 (2): 261-70, 1995. [PUBMED Abstract]
- Poen JC, Hoppe RT, Horning SJ: High-dose therapy and autologous bone marrow transplantation for relapsed/refractory Hodgkin's disease: the impact of involved field radiotherapy on patterns of failure and survival. Int J Radiat Oncol Biol Phys 36 (1): 3-12, 1996. [PUBMED Abstract]
- Milgrom SA, Jauhari S, Plastaras JP, et al.: A multi-institutional analysis of peritransplantation radiotherapy in patients with relapsed/refractory Hodgkin lymphoma undergoing autologous stem cell transplantation. Cancer 123 (8): 1363-1371, 2017. [PUBMED Abstract]
- Moskowitz AJ, Hamlin PA, Perales MA, et al.: Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol 31 (4): 456-60, 2013. [PUBMED Abstract]
- Little R, Wittes RE, Longo DL, et al.: Vinblastine for recurrent Hodgkin's disease following autologous bone marrow transplant. J Clin Oncol 16 (2): 584-8, 1998. [PUBMED Abstract]
- Harker WG, Kushlan P, Rosenberg SA: Combination chemotherapy for advanced Hodgkin's disease after failure of MOPP: ABVD and B-CAVe. Ann Intern Med 101 (4): 440-6, 1984. [PUBMED Abstract]
- Tourani JM, Levy R, Colonna P, et al.: High-dose salvage chemotherapy without bone marrow transplantation for adult patients with refractory Hodgkin's disease. J Clin Oncol 10 (7): 1086-94, 1992. [PUBMED Abstract]
- Canellos GP, Petroni GR, Barcos M, et al.: Etoposide, vinblastine, and doxorubicin: an active regimen for the treatment of Hodgkin's disease in relapse following MOPP. Cancer and Leukemia Group B. J Clin Oncol 13 (8): 2005-11, 1995. [PUBMED Abstract]
- Longo DL, Duffey PL, Young RC, et al.: Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin's disease after combination chemotherapy: the low probability for cure. J Clin Oncol 10 (2): 210-8, 1992. [PUBMED Abstract]
- Ng AK, Li S, Neuberg D, et al.: Comparison of MOPP versus ABVD as salvage therapy in patients who relapse after radiation therapy alone for Hodgkin's disease. Ann Oncol 15 (2): 270-5, 2004. [PUBMED Abstract]
- Specht L, Horwich A, Ashley S: Salvage of relapse of patients with Hodgkin's disease in clinical stages I or II who were staged with laparotomy and initially treated with radiotherapy alone. A report from the international database on Hodgkin's disease. Int J Radiat Oncol Biol Phys 30 (4): 805-11, 1994. [PUBMED Abstract]
- Horwich A, Specht L, Ashley S: Survival analysis of patients with clinical stages I or II Hodgkin's disease who have relapsed after initial treatment with radiotherapy alone. Eur J Cancer 33 (6): 848-53, 1997. [PUBMED Abstract]
- Uematsu M, Tarbell NJ, Silver B, et al.: Wide-field radiation therapy with or without chemotherapy for patients with Hodgkin disease in relapse after initial combination chemotherapy. Cancer 72 (1): 207-12, 1993. [PUBMED Abstract]
- Josting A, Nogová L, Franklin J, et al.: Salvage radiotherapy in patients with relapsed and refractory Hodgkin's lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol 23 (7): 1522-9, 2005. [PUBMED Abstract]
- Herrera AF, Moskowitz AJ, Bartlett NL, et al.: Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 131 (11): 1183-1194, 2018. [PUBMED Abstract]
Treatment of Nodular Lymphocyte–Predominant HL (NLPHL)
Immunophenotypic differences distinguish NLPHL (CD15-, CD20+, CD30-) from lymphocyte-rich classic Hodgkin lymphoma (HL) (CD15+, CD20-, CD30+).[1,2] The largest retrospective report of 426 cases showed no significant difference in clinical response or outcome to standard therapies for these two subgroups when patients present with early-stage disease (stage I or II).[3][Level of evidence C1]
Patients with NLPHL have earlier-stage disease and longer survival than those with classic HL.[4,5] NLPHL is usually diagnosed in asymptomatic younger patients with cervical or inguinal lymph nodes; this usually occurs without mediastinal involvement. Unlike patients with classic HL, bulky disease, B symptoms, and contiguous spread are uncommon in patients with NLPHL.[6,7] An international prognostic score identified age 45 years or older, stage III or IV disease, hemoglobin less than 10.5 g/dL, and splenic involvement as poor prognostic factors for NLPHL.[8]
Treatment Options for NLPHL
Treatment options for NLPHL include:
Watchful waiting/active surveillance
Because of the favorable prognosis for NLPHL and the potential long-term side effects of therapy, studies have evaluated watchful waiting or active surveillance for patients with asymptomatic, low tumor burden disease.[9] In a retrospective comparison, 37 such patients managed with active surveillance had a 5-year progression-free survival (PFS) rate of 77%, versus 85% for patients receiving active treatment.[10][Level of evidence C3]
Radiation therapy
Limited-field radiation therapy is the most-common treatment approach for patients with early-stage disease. This histology is rare, but this approach is based on retrospective analysis spanning several decades.[5,11-15]
Patients with nonbulky lymphocyte–predominant disease presenting in unilateral high neck (above the thyroid notch) or epitrochlear locations require only involved-field radiation therapy (IFRT) after clinical staging.[16] A retrospective report of 426 cases of lymphocyte-predominant HL (including the nodular lymphocyte–predominant and lymphocyte-rich classic subtypes) showed that more patients died of acute and long-term treatment-related toxicity than of recurrent HL.[3][Level of evidence C1] Limitation of radiation dose and radiation fields and avoidance of leukemogenic chemotherapeutic agents, along with watchful waiting policies, should be investigated for these subgroups.[15,17]
Chemotherapy
For patients with early-stage NLPHL, ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) for two to three cycles has been combined with IFRT on the basis of anecdotal single-arm trials.[5,18]
For patients with advanced-stage NLPHL, chemotherapy regimens designed for patients with non-Hodgkin lymphomas, such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone), may be preferred, based on two retrospective reviews and a phase II study.[7,19-21][Level of evidence C3]
Rituximab
In a phase II trial of 39 patients with previously untreated and relapsed NLPHL, most of whom had advanced-stage disease, treatment with rituximab yielded a 100% response rate. With a median follow-up of 9.8 years, the median PFS was 3.0 years for patients who received rituximab induction only and 5.6 years for patients who received rituximab induction plus rituximab maintenance.[22][Level of evidence C2] With induction only, 9 of 23 patients had disease relapse with an aggressive B-cell lymphoma.
Follow-Up
Despite a usually favorable prognosis, there is a tendency for histological transformation of NLPHL to diffuse large B-cell lymphoma or T-cell–rich large B-cell lymphoma in approximately 10% of patients by 10 years.[6,22,23] This propensity of NLPHL to transform to aggressive B-cell lymphoma underscores the importance of long-term follow-up and rebiopsy at relapse.[22,24]
With a median follow-up of 7 to 8 years, more patients died of treatment-related toxic effects (acute and long-term) than of recurrent HL. Limitation of radiation dose and fields and avoidance of leukemogenic chemotherapeutic agents, along with watchful waiting policies, should be investigated for these subgroups.[5,17,25]
The treatment approach for relapsing disease is similar to that for recurrent follicular lymphoma. Based on age and performance status, some patients receive sequential therapies and watchful waiting, and some patients receive aggressive salvage chemoimmunotherapy (like R-ICE [rituximab, ifosfamide, carboplatin, and etoposide]) followed by stem cell transplant.[7,26,27]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Harris NL: Hodgkin's lymphomas: classification, diagnosis, and grading. Semin Hematol 36 (3): 220-32, 1999. [PUBMED Abstract]
- Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al.: Lymphocyte-rich classical Hodgkin's lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin's Study Group trials. J Clin Oncol 23 (24): 5739-45, 2005. [PUBMED Abstract]
- Diehl V, Sextro M, Franklin J, et al.: Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin's disease and lymphocyte-rich classical Hodgkin's disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin's Disease. J Clin Oncol 17 (3): 776-83, 1999. [PUBMED Abstract]
- Nogová L, Reineke T, Brillant C, et al.: Lymphocyte-predominant and classical Hodgkin's lymphoma: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol 26 (3): 434-9, 2008. [PUBMED Abstract]
- Eichenauer DA, Plütschow A, Fuchs M, et al.: Long-Term Course of Patients With Stage IA Nodular Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the German Hodgkin Study Group. J Clin Oncol 33 (26): 2857-62, 2015. [PUBMED Abstract]
- Eichenauer DA, Plütschow A, Fuchs M, et al.: Long-Term Follow-Up of Patients With Nodular Lymphocyte-Predominant Hodgkin Lymphoma Treated in the HD7 to HD15 Trials: A Report From the German Hodgkin Study Group. J Clin Oncol 38 (7): 698-705, 2020. [PUBMED Abstract]
- Bartlett NL: Treatment of Nodular Lymphocyte Hodgkin Lymphoma: The Goldilocks Principle. J Clin Oncol 38 (7): 662-668, 2020. [PUBMED Abstract]
- Binkley MS, Flerlage JE, Savage KJ, et al.: International Prognostic Score for Nodular Lymphocyte-Predominant Hodgkin Lymphoma. J Clin Oncol 42 (19): 2271-2280, 2024. [PUBMED Abstract]
- Moskowitz AJ: NLP Hodgkin lymphoma: can we get away with less? Blood 135 (26): 2329-2330, 2020. [PUBMED Abstract]
- Borchmann S, Joffe E, Moskowitz CH, et al.: Active surveillance for nodular lymphocyte-predominant Hodgkin lymphoma. Blood 133 (20): 2121-2129, 2019. [PUBMED Abstract]
- Chen RC, Chin MS, Ng AK, et al.: Early-stage, lymphocyte-predominant Hodgkin's lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol 28 (1): 136-41, 2010. [PUBMED Abstract]
- Nogová L, Reineke T, Eich HT, et al.: Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin's lymphoma: a retrospective analysis from the German Hodgkin Study Group (GHSG). Ann Oncol 16 (10): 1683-7, 2005. [PUBMED Abstract]
- Wilder RB, Schlembach PJ, Jones D, et al.: European Organization for Research and Treatment of Cancer and Groupe d'Etude des Lymphomes de l'Adulte very favorable and favorable, lymphocyte-predominant Hodgkin disease. Cancer 94 (6): 1731-8, 2002. [PUBMED Abstract]
- Eichenauer DA, Engert A: How I treat nodular lymphocyte-predominant Hodgkin lymphoma. Blood 136 (26): 2987-2993, 2020. [PUBMED Abstract]
- Binkley MS, Rauf MS, Milgrom SA, et al.: Stage I-II nodular lymphocyte-predominant Hodgkin lymphoma: a multi-institutional study of adult patients by ILROG. Blood 135 (26): 2365-2374, 2020. [PUBMED Abstract]
- Russell KJ, Hoppe RT, Colby TV, et al.: Lymphocyte predominant Hodgkin's disease: clinical presentation and results of treatment. Radiother Oncol 1 (3): 197-205, 1984. [PUBMED Abstract]
- Aster JC: Lymphocyte-predominant Hodgkin's disease: how little therapy is enough? J Clin Oncol 17 (3): 744-6, 1999. [PUBMED Abstract]
- Savage KJ, Skinnider B, Al-Mansour M, et al.: Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood 118 (17): 4585-90, 2011. [PUBMED Abstract]
- Canellos GP, Mauch P: What is the appropriate systemic chemotherapy for lymphocyte-predominant Hodgkin's lymphoma? J Clin Oncol 28 (1): e8, 2010. [PUBMED Abstract]
- Xing KH, Connors JM, Lai A, et al.: Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood 123 (23): 3567-73, 2014. [PUBMED Abstract]
- Fanale MA, Cheah CY, Rich A, et al.: Encouraging activity for R-CHOP in advanced stage nodular lymphocyte-predominant Hodgkin lymphoma. Blood 130 (4): 472-477, 2017. [PUBMED Abstract]
- Advani RH, Horning SJ, Hoppe RT, et al.: Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 32 (9): 912-8, 2014. [PUBMED Abstract]
- Al-Mansour M, Connors JM, Gascoyne RD, et al.: Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin's lymphoma. J Clin Oncol 28 (5): 793-9, 2010. [PUBMED Abstract]
- Kenderian SS, Habermann TM, Macon WR, et al.: Large B-cell transformation in nodular lymphocyte-predominant Hodgkin lymphoma: 40-year experience from a single institution. Blood 127 (16): 1960-6, 2016. [PUBMED Abstract]
- Pellegrino B, Terrier-Lacombe MJ, Oberlin O, et al.: Lymphocyte-predominant Hodgkin's lymphoma in children: therapeutic abstention after initial lymph node resection--a Study of the French Society of Pediatric Oncology. J Clin Oncol 21 (15): 2948-52, 2003. [PUBMED Abstract]
- Eichenauer DA, Plütschow A, Schröder L, et al.: Relapsed and refractory nodular lymphocyte-predominant Hodgkin lymphoma: an analysis from the German Hodgkin Study Group. Blood 132 (14): 1519-1525, 2018. [PUBMED Abstract]
- Akhtar S, Montoto S, Boumendil A, et al.: High dose chemotherapy and autologous stem cell transplantation in nodular lymphocyte-predominant Hodgkin lymphoma: A retrospective study by the European society for blood and marrow transplantation-lymphoma working party. Am J Hematol 93 (1): 40-46, 2018. [PUBMED Abstract]
Latest Updates to This Summary (02/12/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Hodgkin Lymphoma
Updated statistics with estimated new cases and deaths for 2025 (cited American Cancer Society as reference 1).
Treatment Option Overview for Hodgkin Lymphoma (HL)
Revised Table 3, Treatment Options for Hodgkin Lymphoma, to list chemotherapy with or without nivolumab or brentuximab vedotin as a treatment option for advanced classic HL.
Revised Table 4, Chemotherapy Regimens Used to Treat Hodgkin Lymphoma, to list advanced classic HL as a prognostic group that is treated with the ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) regimen.
Treatment of Advanced Classic HL
Revised text to list chemotherapy plus immunotherapy or an antibody-drug conjugate as a treatment option for advanced classic HL.
Revised text about the results of a randomized prospective trial of 994 patients with previously untreated advanced-stage HL that compared BV-AVD (brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine) with N-AVD (nivolumab plus doxorubicin, vinblastine, and dacarbazine) (cited Herrera et al. as reference 4 and level of evidence B1).
Added text to state that, for patients with advanced-stage HL, six cycles of N-AVD is now the standard approach. In situations where immunotherapy might be contraindicated (such as active vasculitis or inflammatory colitis), BV-AVD is a good option. When the financial toxicity of N-AVD or BV-AVD precludes their use (such as in a nation with constrained health care options), ABVD is still a reasonable and cost-effective approach.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult Hodgkin lymphoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewer for Hodgkin Lymphoma Treatment is:
- Eric J. Seifter, MD (Johns Hopkins University)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Hodgkin Lymphoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lymphoma/hp/adult-hodgkin-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389473]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.